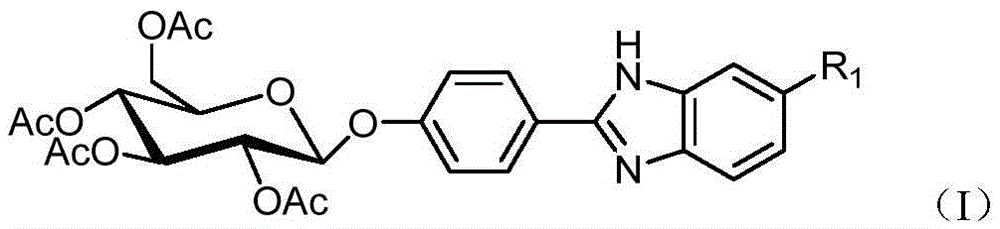
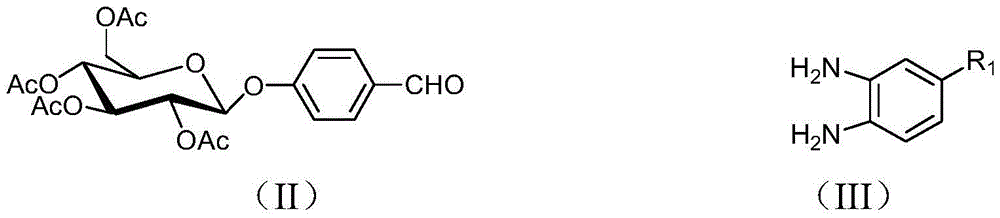
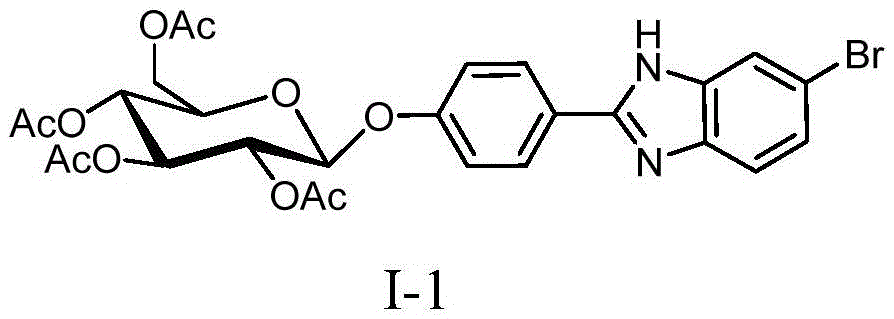
Glycosyl benzimidazole compound and preparation method as well as application thereof
A technology based on benzimidazole and benzimidazole, which is applied in the application field of preparing antibacterial drugs, can solve problems such as the difficulty of sugar-based nitrogen-containing heterocyclic compounds, and achieve good antibacterial activity, good water solubility, and high reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] 1. Dissolve 452 mg of 4-glucopyranose benzaldehyde and 186 mg of 4-bromo-o-phenylenediamine represented by formula (II) in 20 mL of DMF, add 5 mL of water, heat up to 80° C., and keep warm for 12 hours under magnetic stirring. After the reaction was completed, cool down to room temperature, add 30 mL of water, stir for 30 min, then add 30 mL of ethyl acetate for extraction. Use a separatory funnel to separate the liquid, add 30 mL of ethyl acetate to the aqueous phase for extraction, combine the organic phase after liquid separation, wash the organic phase with 50 mL of water 3 times, separate the liquid, add anhydrous magnesium sulfate to the organic phase to dry, filter, and spin dry the reaction solution , to obtain 587 mg of a new glycosylbenzimidazole compound represented by formula (I-1), with a yield of 95%. The compound of formula (I-1) is characterized as follows: yellow syrup, 1 HNMR (500MHz, CDCl 3 -d 6 )δ=7.97(d,J=7.8Hz,2H,ArH),7.71(s,1H,ArH),...
Embodiment 2
[0030] 1. Dissolve 452 mg of 4-glucopyranose benzaldehyde and 186 mg of 4-bromo-o-phenylenediamine represented by formula (II) in 20 mL of DMF, add 3 mL of water, heat up to 75° C., and keep warm for 14 hours under magnetic stirring. After the reaction was completed, cool down to room temperature, add 30 mL of water, stir for 30 min, then add 30 mL of ethyl acetate for extraction. Use a separatory funnel to separate the liquid, add 30 mL of ethyl acetate to the aqueous phase for extraction, combine the organic phase after liquid separation, wash the organic phase with 50 mL of water 3 times, separate the liquid, add anhydrous magnesium sulfate to the organic phase to dry, filter, and spin dry the reaction solution , to obtain 574 mg of a new glycosylbenzimidazole compound represented by formula (I-1), with a yield of 93%.
[0031] 2. Preparation of Bacterial Suspension
[0032] Under aseptic conditions, use the broth medium to activate Staphylococcus aureus and Escherichia co...
Embodiment 3
[0038]
[0039] 1. Replace 186 mg of 4-bromo-o-phenylenediamine with 142 mg of 4-chloro-o-phenylenediamine, and other conditions are the same as in Example 1. 533 mg of the new glycosyl benzimidazole compound represented by the formula (I-2) was obtained with a yield of 93%. Compound (I-2) was characterized as follows: yellow syrup, 1 HNMR (500MHz, CDCl 3 -d 6 )δ=7.97(d,J=8.8Hz,2H,ArH),7.54(s,1H,ArH),7.49(d,J=8.6Hz,1H,ArH),7.21–7.19(m,1H,ArH) ,7.00(d,J=8.8Hz,2H,ArH),5.30(t,J=8.0Hz,2H,GH),5.20–5.14(m,1H,GH),5.12(d,J=7.4Hz,1H ,GH),4.30–4.26(m,1H,GH),4.19–4.16(m,1H,GH),3.90–3.87(m,1H,GH),2.05–2.00(m,12H,AcH). 13 CNMR (126MHz, CDCl3 -d 6 )δ=170.69, 170.23, 169.52, 169.46, 162.85, 158.24, 152.58, 128.37, 128.20, 124.48, 123.24, 117.13, 98.42, 72.59, 72.06, 71.08, 68.18, 64.747–ES + ):found[M+H] + 575.1449.C 27 h 27 ClN 2 o 10 requires[M+H] + 575.1427.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap