Chromene derivatives as inhibitors of TCR-NCK interaction
A C1-C4, C3-C6 technology, applied in the direction of medical preparations containing active ingredients, anti-inflammatory agents, drug combinations, etc., can solve the lack of specific side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
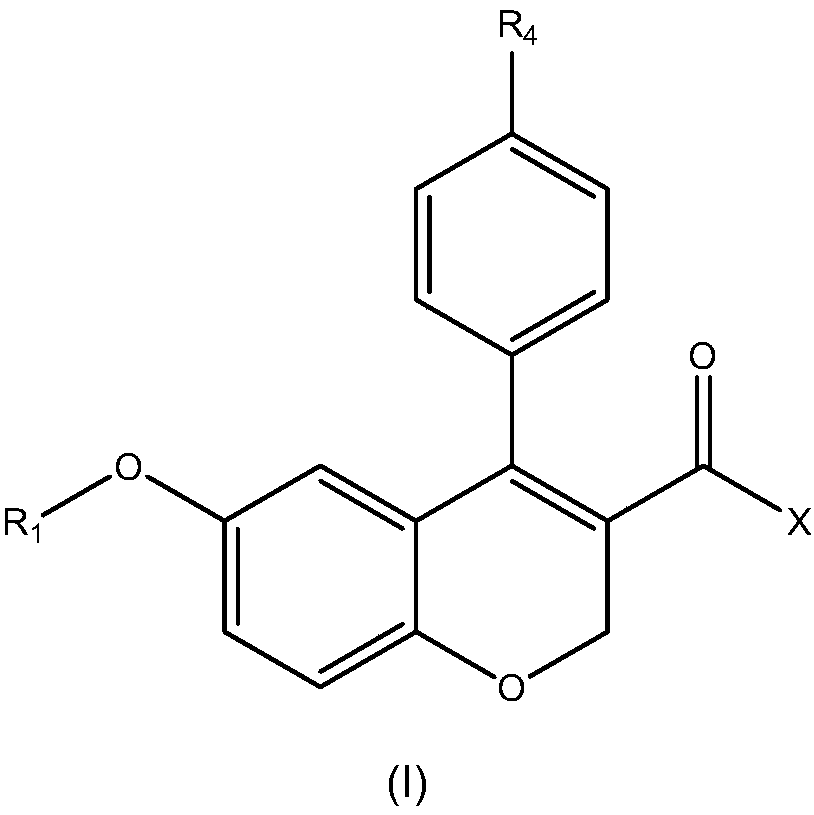
[0083] Example 1: Synthesis of the compound of the present invention
[0084] Synthesis scheme of AX-105 to AX-108
[0085]
[0086] Synthesis of compound AX-137: compound 1 (3g, 1 equivalent), AgNO 3 A mixture of a solution of ethanol (30 ml) (3.5 g, 2 equivalents) and NaOH (1.7 g, 4 equivalents) dissolved in water (30 ml) was refluxed at 85°C and stirred at this temperature for 4 hours. The reaction was monitored by TLC. After completion, the mixture was acidified with 1M HCl and extracted with DCM (2×30 ml). The combined organic layer was washed with water (20 ml), brine solution (10 ml), and dried over anhydrous sodium sulfate. The organic layer was evaporated under reduced pressure to yield 1.5 g of the desired product with a purity of 96.2% by HPLC. 1 H NMR(CDCl 3 )δ7.17-7.08(m, 4H), 6.89-6.87(d, 1H), 6.84-6.81(m, 1H), 6.21-6.20(d, 1H), 4.96(s, 2H), 3.61(s, 3H). For C 17 H 13 FO 4 , Theoretical MS: 300.28; M + +1 Actual value: 301.0.
[0087] Synthesis of compound AX-105:...
Embodiment 2
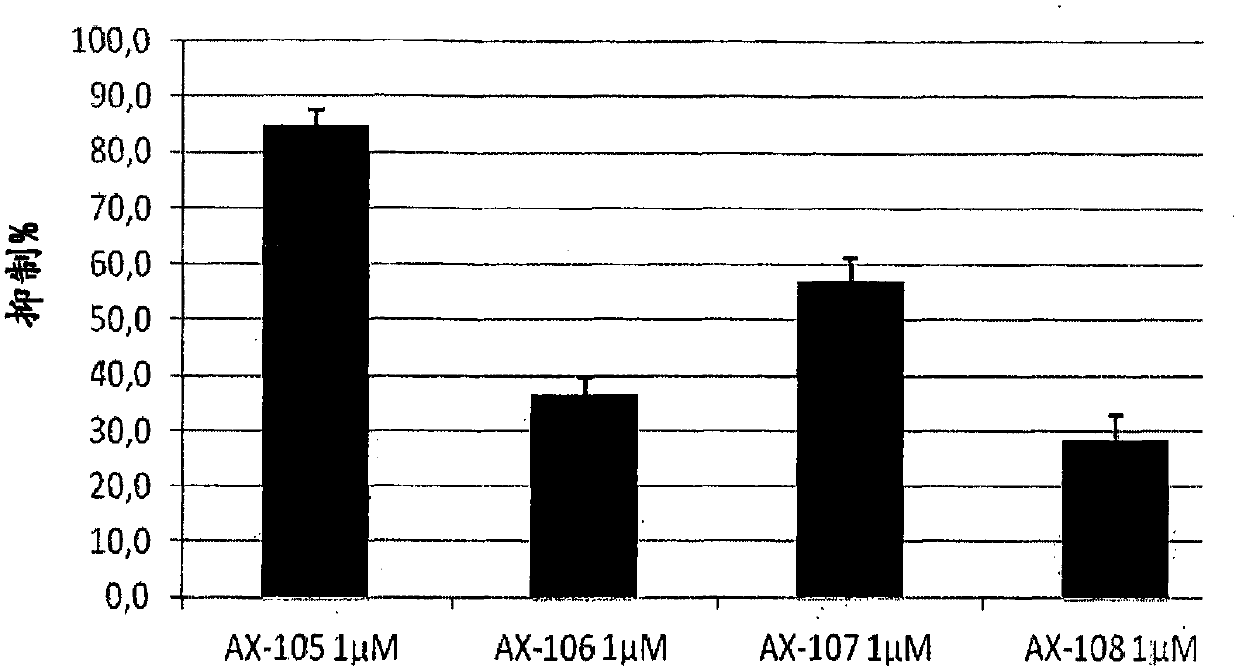
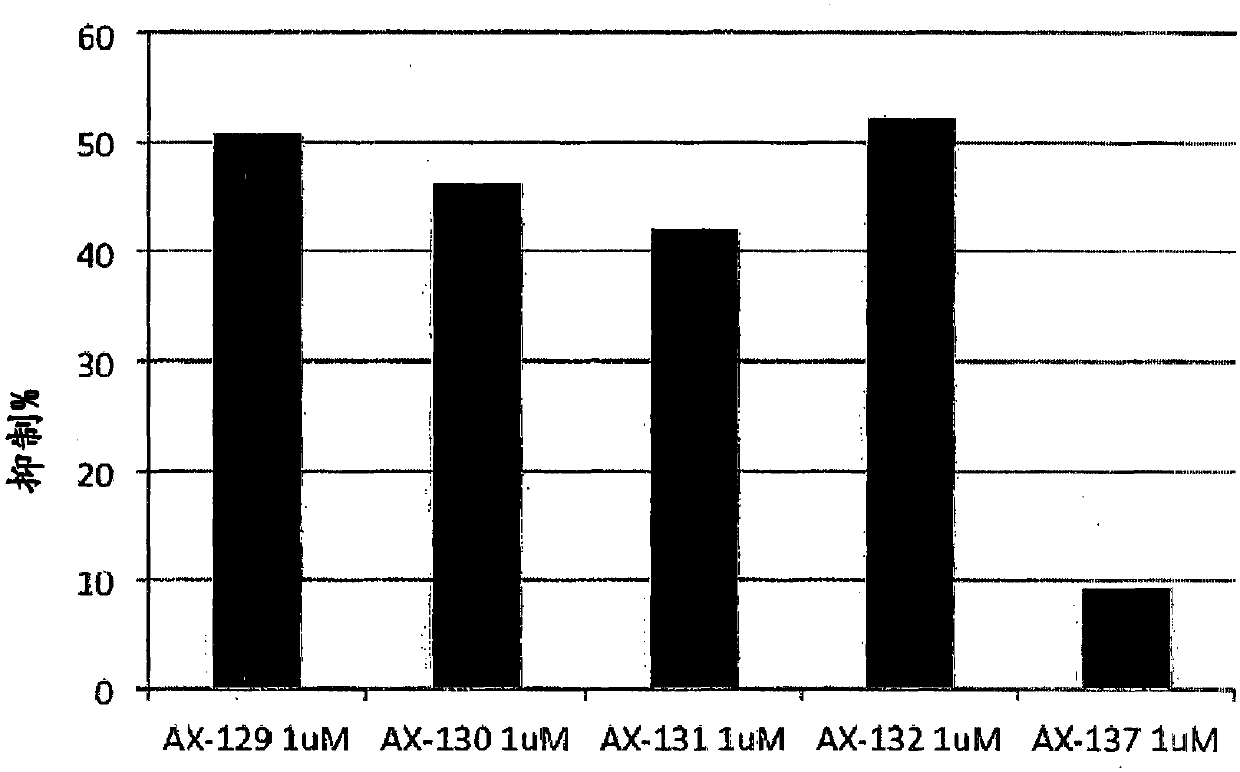
[0105] Example 2: Inhibition of T cell proliferation induced by TCR stimulation
[0106] Using primitive T lymphocytes (PBMC, peripheral blood mononuclear cells) obtained from the blood of healthy human donors, the ability of compounds AX-105, AX-106, AX-107 and AX-108 to induce the proliferation of T lymphocytes by TCR The impact was evaluated. Volunteers' PBMCs were separated by centrifugation of venous blood with Ficoll-Paque Plus density gradient. The purified cells (NWT; Nylon Wood T cells) were plated in a 96-well plate (0.5×10 5 / Well) was cultured with 200 μl of complete medium, and stimulated with OKT3 (10 μg / ml) or OKT3 (1 μg / ml) + CD28 in the presence or absence of different compounds at concentrations of 1 and 10 μM. The culture was incubated for 3 days, and after adding 0.5uCi[3H]TdR / well, the culture was analyzed for the last 12 hours of culture. The radioactivity incorporated into the DNA is determined by liquid scintillation counting. As the cells divide, radio...
Embodiment 3
[0109] Example 3: In vivo testing of type 1 diabetes model
[0110] The development of diabetes is monitored daily, and the development is recorded as positive if the level of glucose in urine is higher than 250 mg / dl in two consecutive measurements performed daily for the RIP-MOVA model or once every two weeks for the NOD model. The effect of treatment on disease incidence and survival rate was evaluated in the RIP-MOVA model as used for insulitis. The histopathological evaluation of H&E insulitis of the sections immersed in paraffin and fixed in formalin was evaluated by blind test using the following classification system: grade 0, non-invasive; grade 1, periinsulitis; 2 Level, intrusion> 25%; level 3, invasion> 75%; and level 4, the remaining islets. In the second stage, the therapeutic effect of the compound was evaluated by treating RIP-mOVA mice that had developed diabetes. During this second phase, the compounds were tested in the NOD mouse model.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com