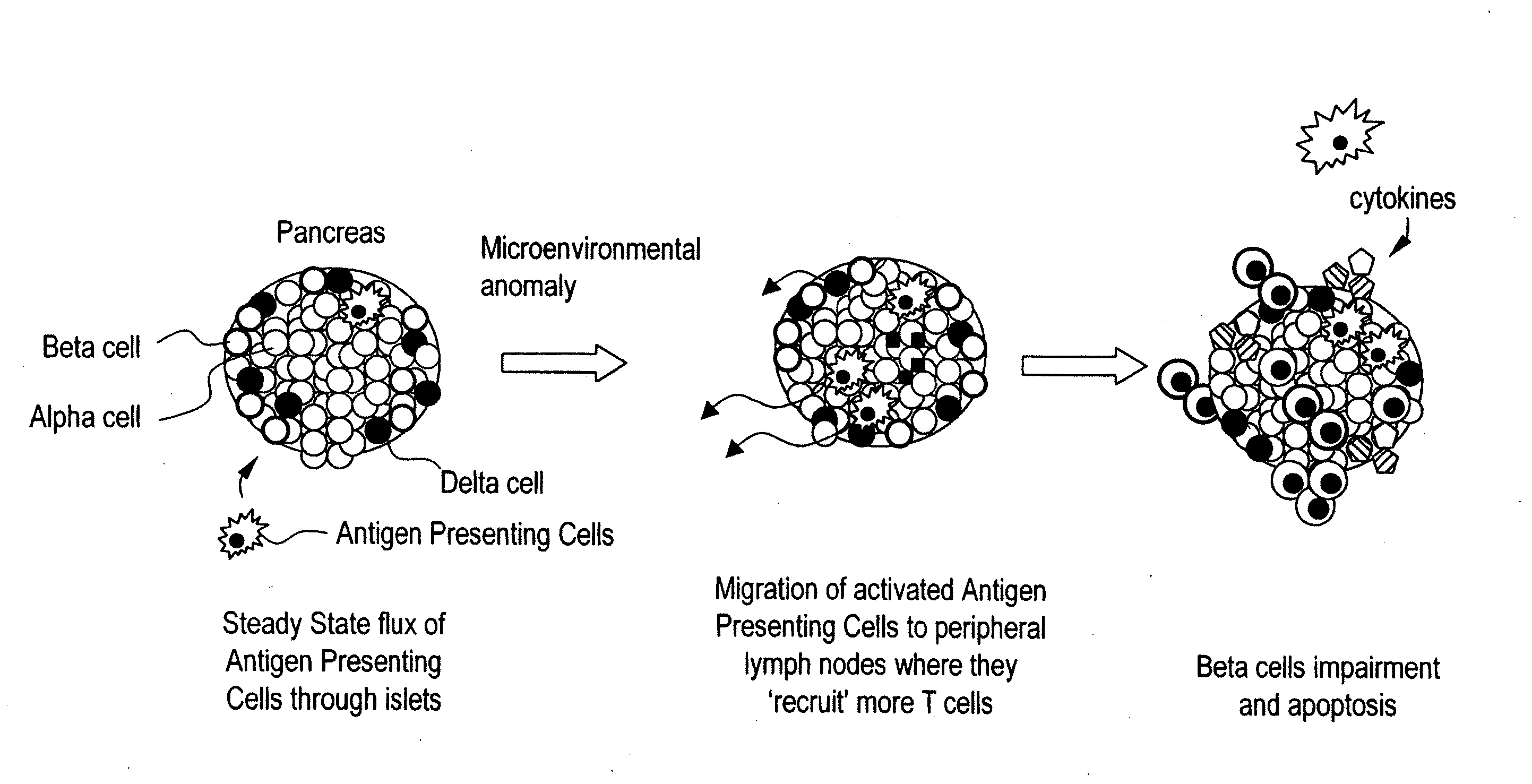
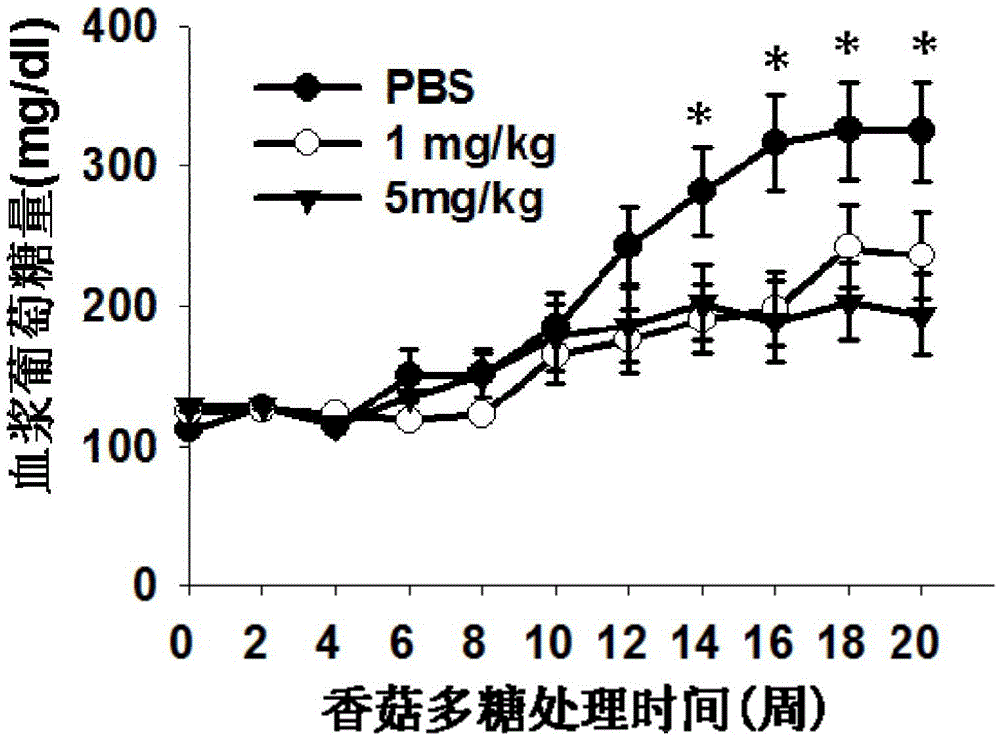
Patents
Literature
35 results about "Nod mouse" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Delivery of as-oligonucleotide microspheres to induce dendritic cell tolerance for the treatment of autoimmune type 1 diabetes
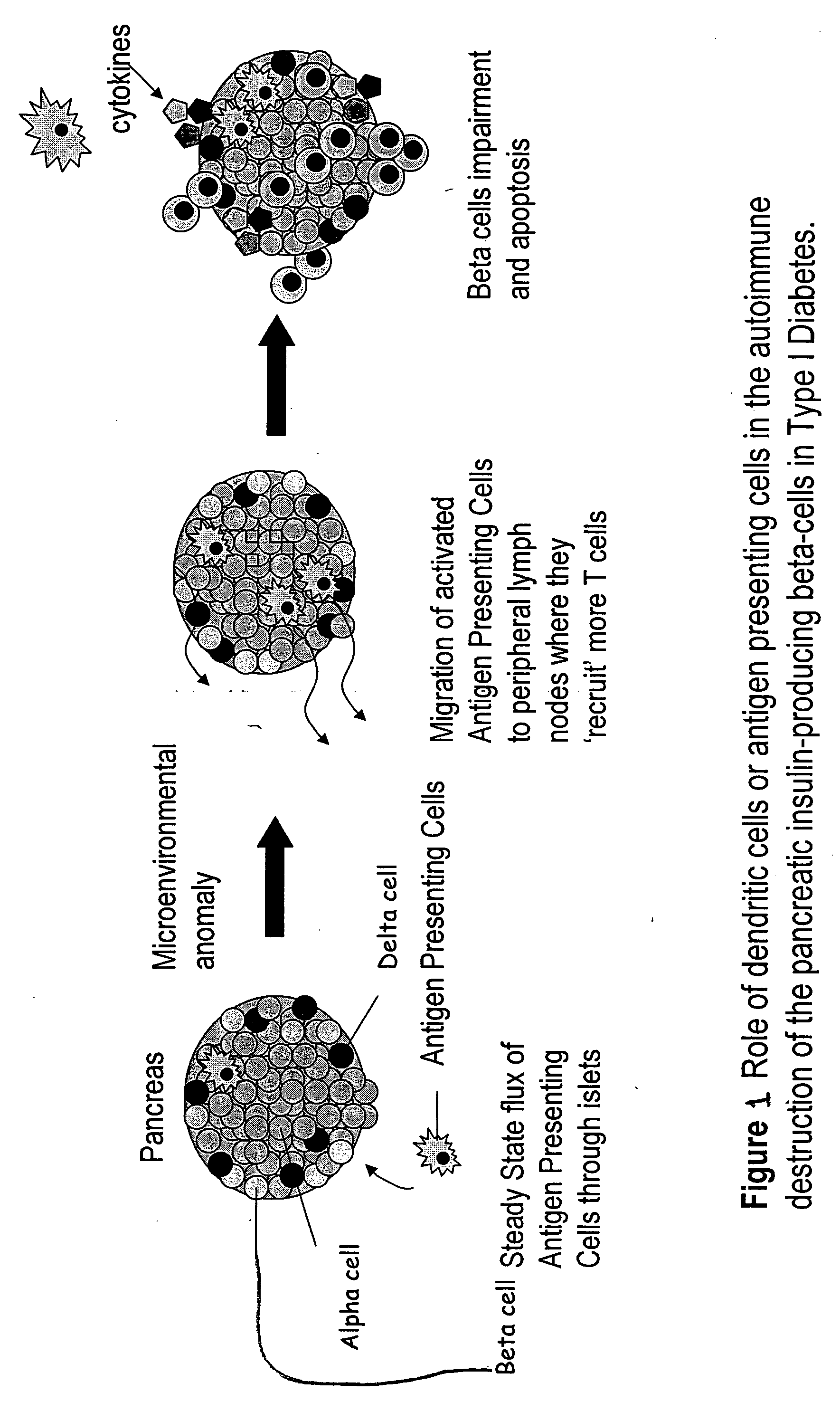
AS-oligonucleotides are delivered in microsphere form in order to induce dendritic cell tolerance, particularly in the non-obese-diabetic (NOD) mouse model. The microspheres incorporate antisense (AS) oligonucleotides. A process includes using an antisense approach to prevent an autoimmune diabetes condition in NOD mice in vivo and in situ. The oligonucleotides are targeted to bind to primary transcripts CD40, CD80, CD86 and their combinations.
Owner:BAXTER INT INC +2
Compositions and methods for preserving insulin-producing cells and insulin production and treating diabetes
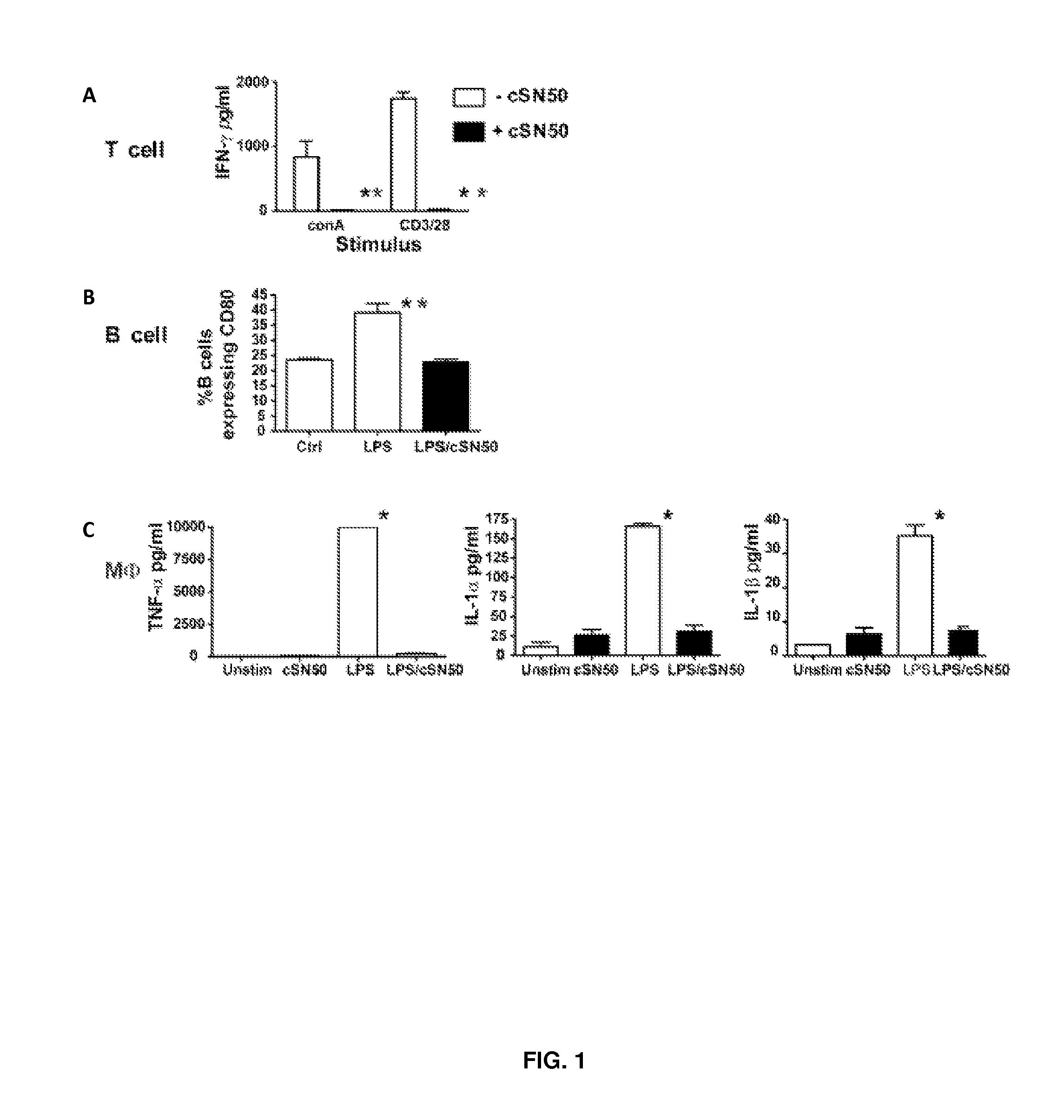
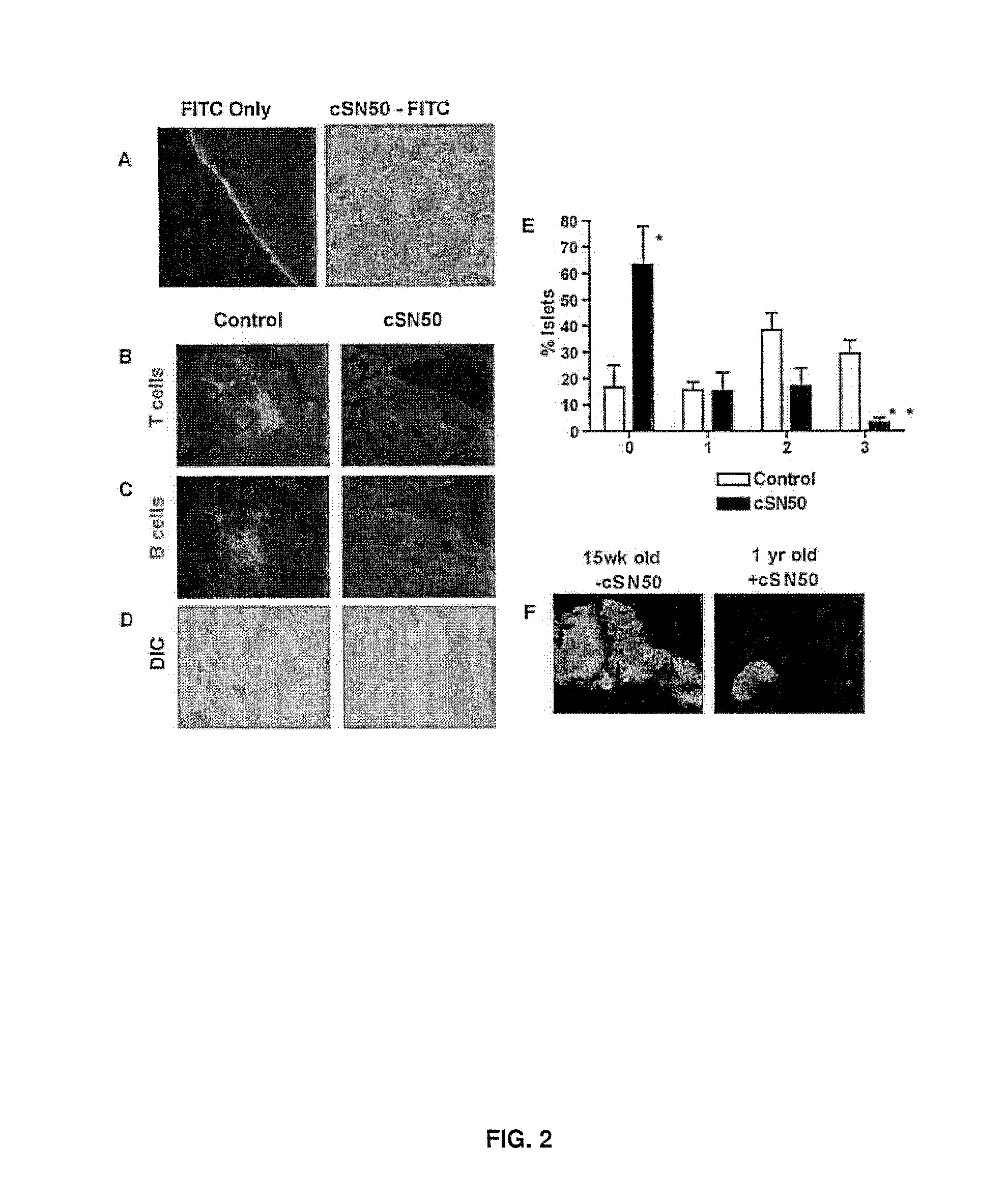
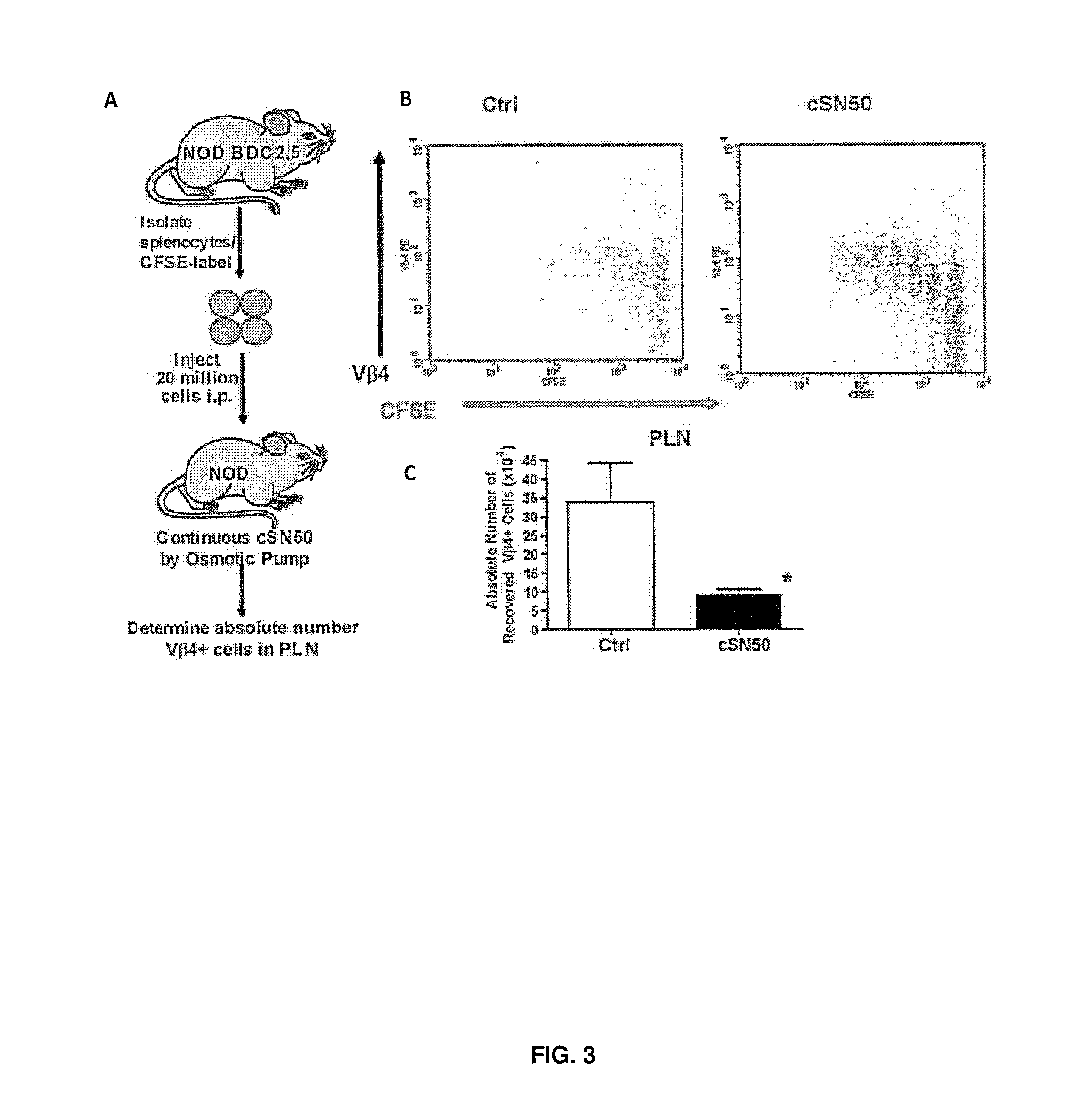
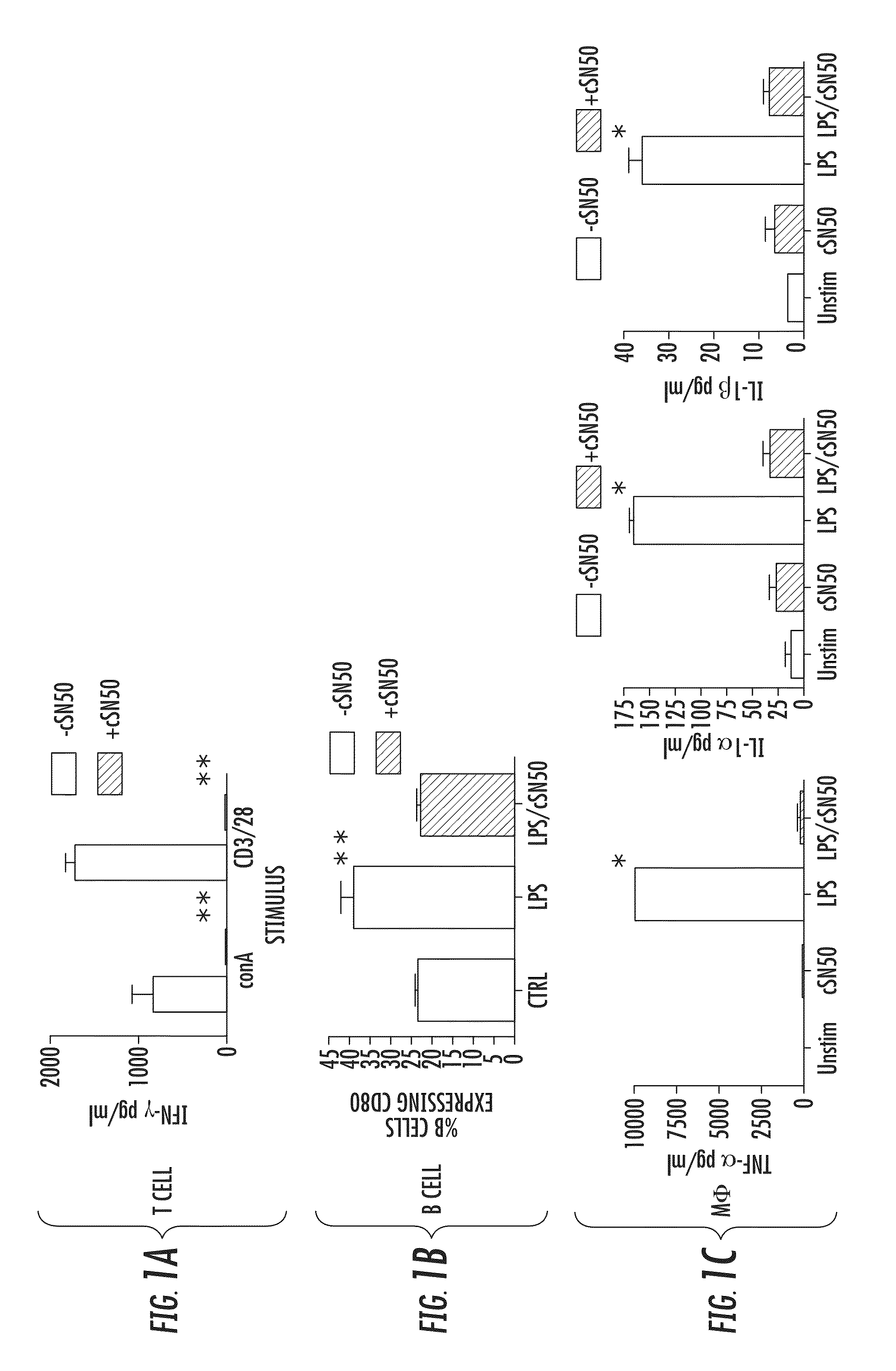
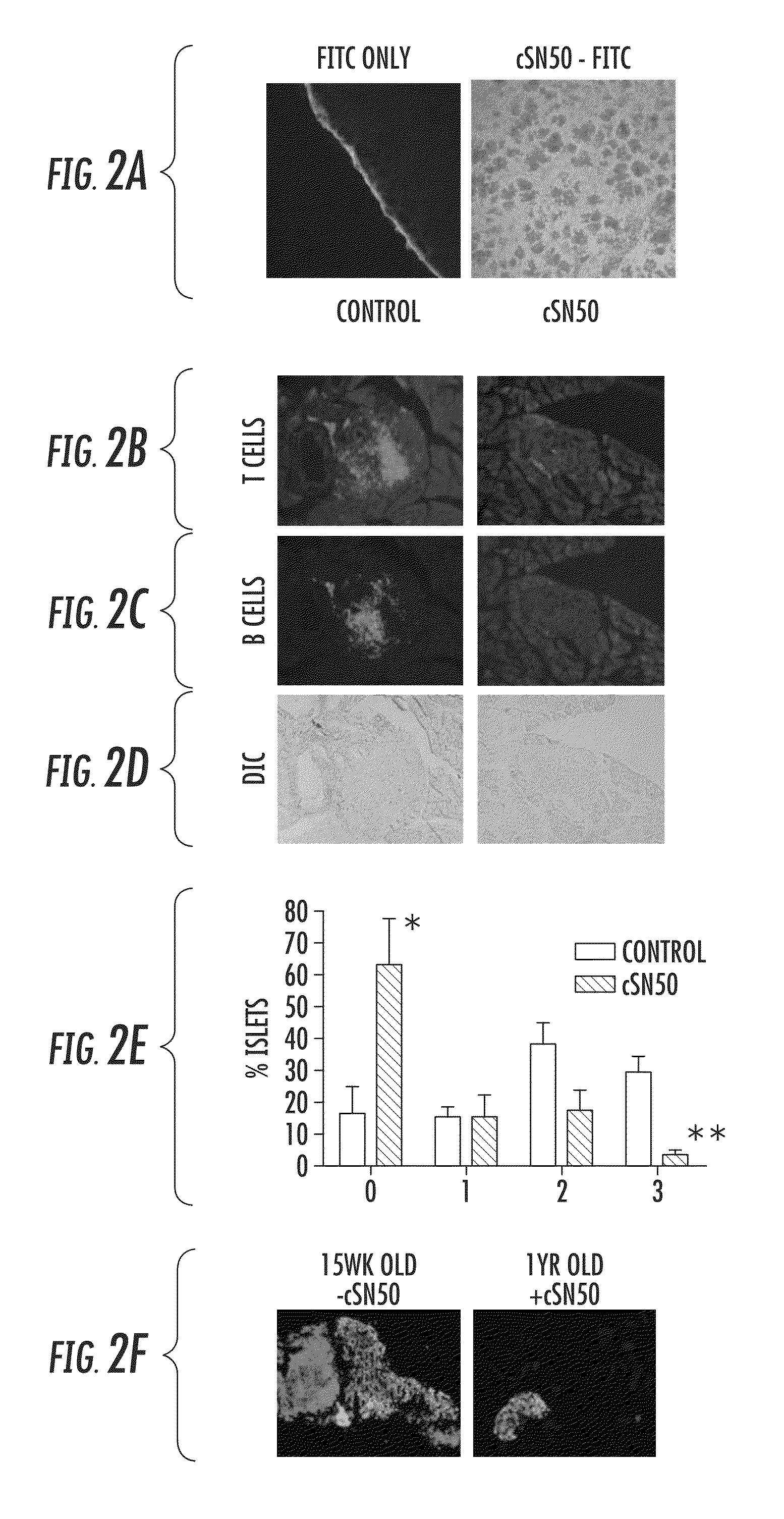
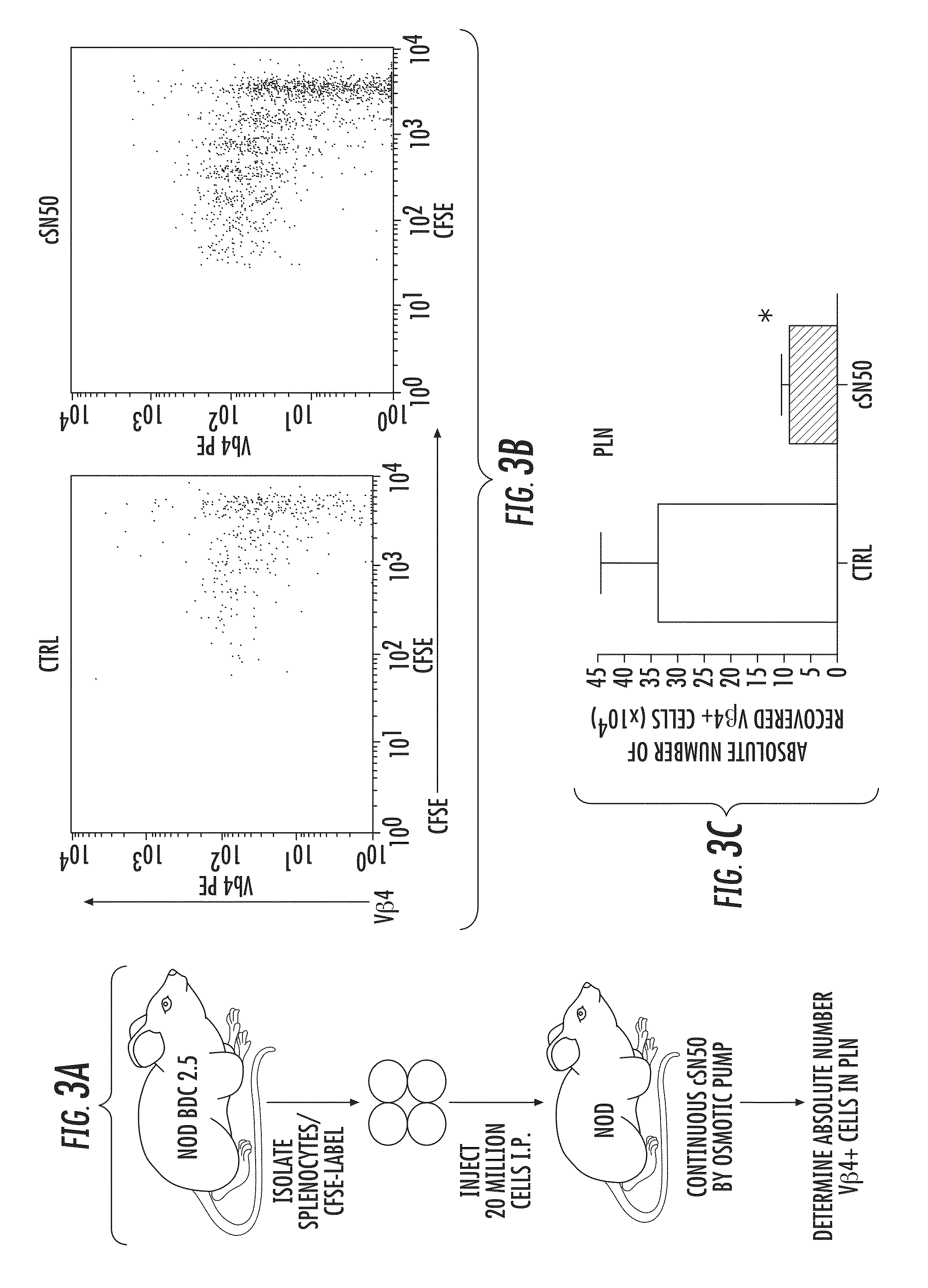
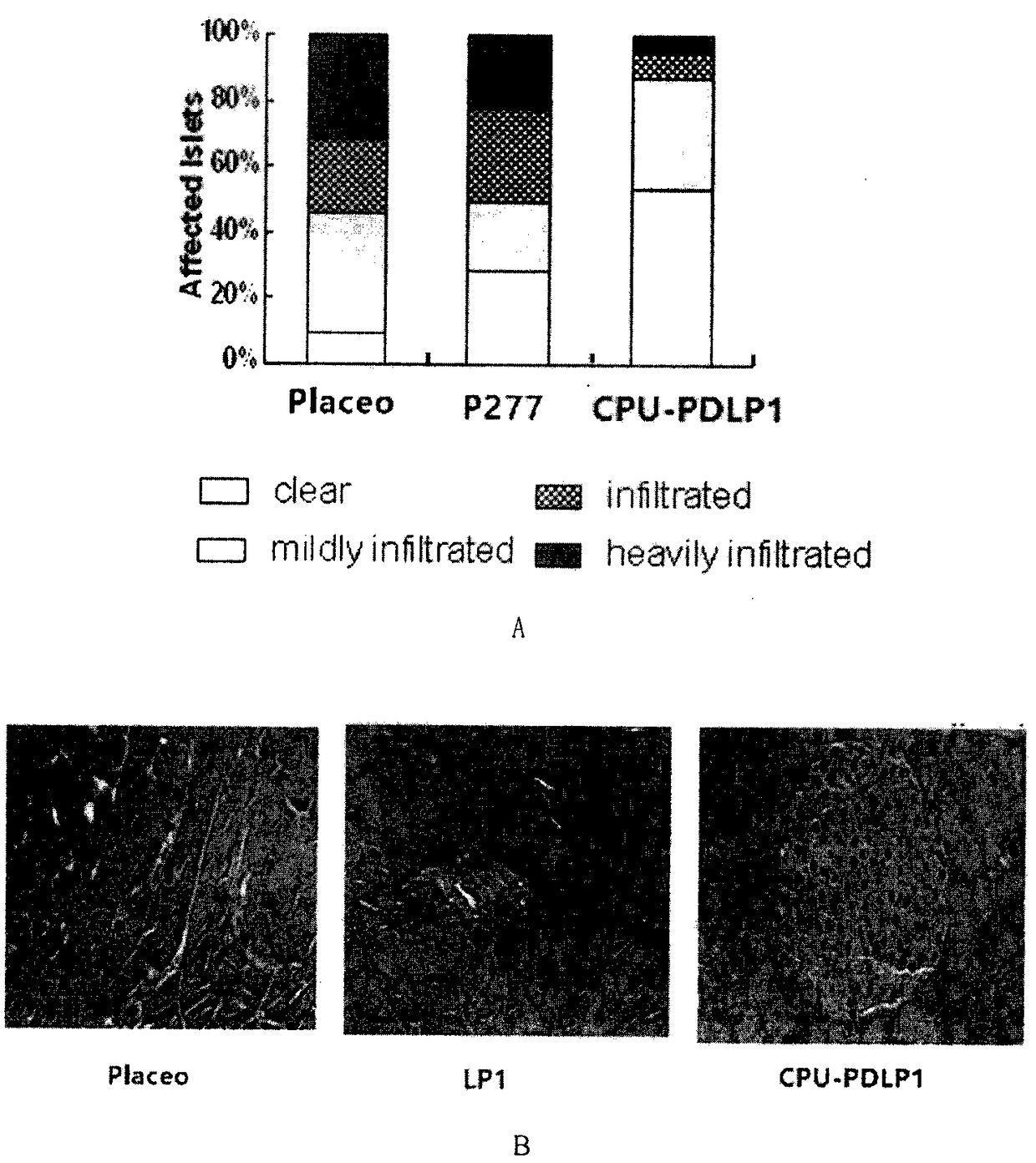
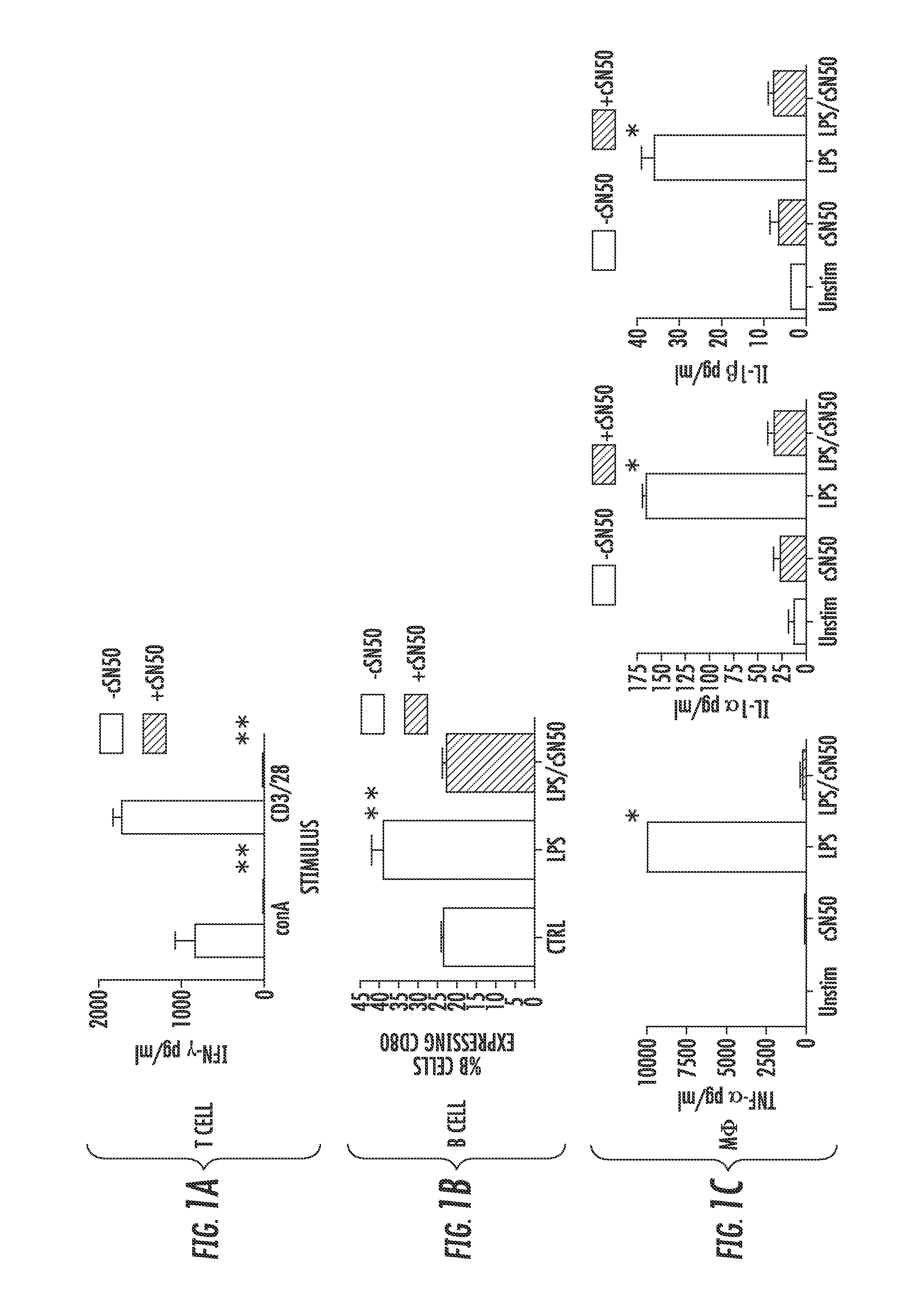
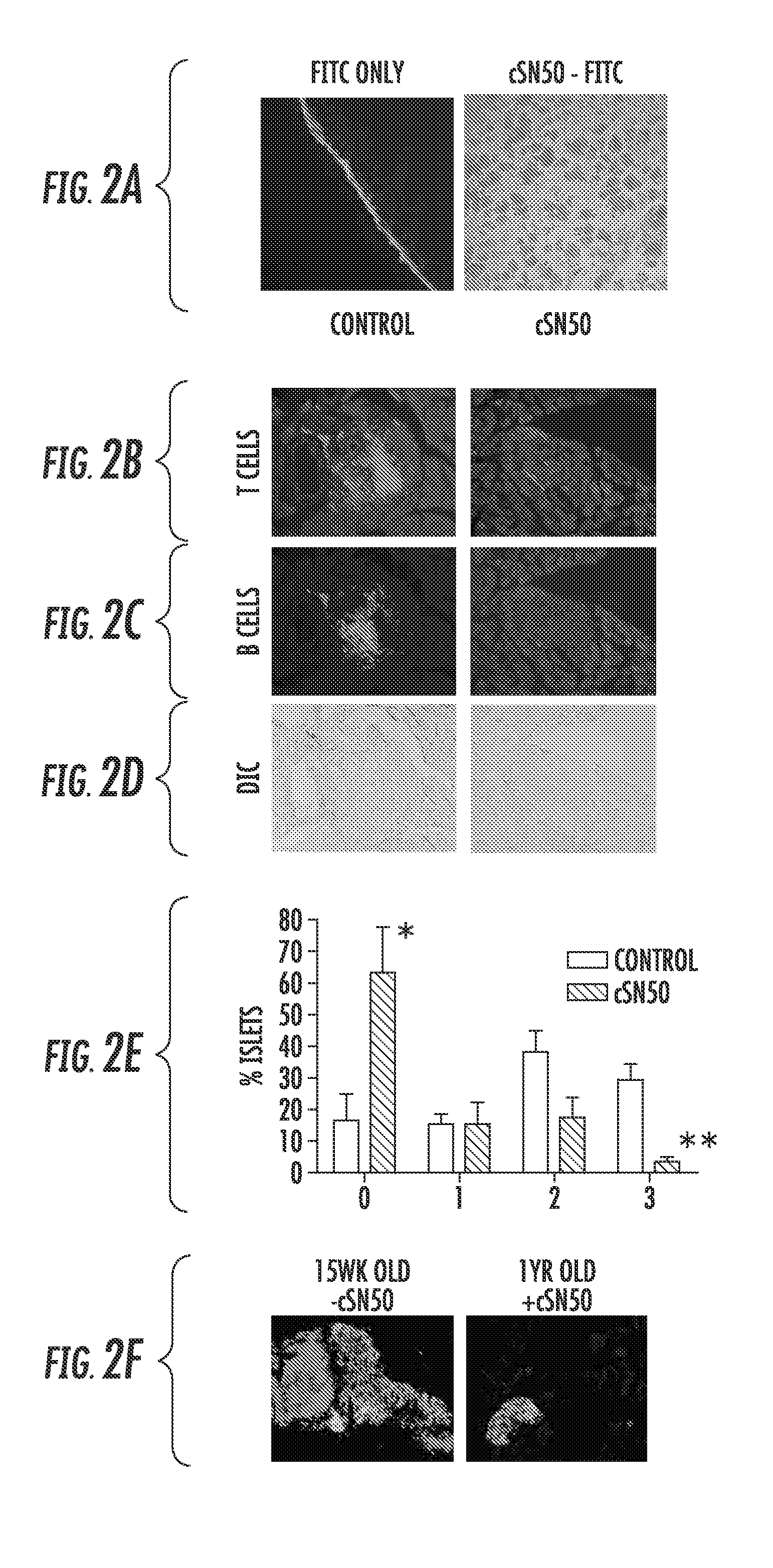
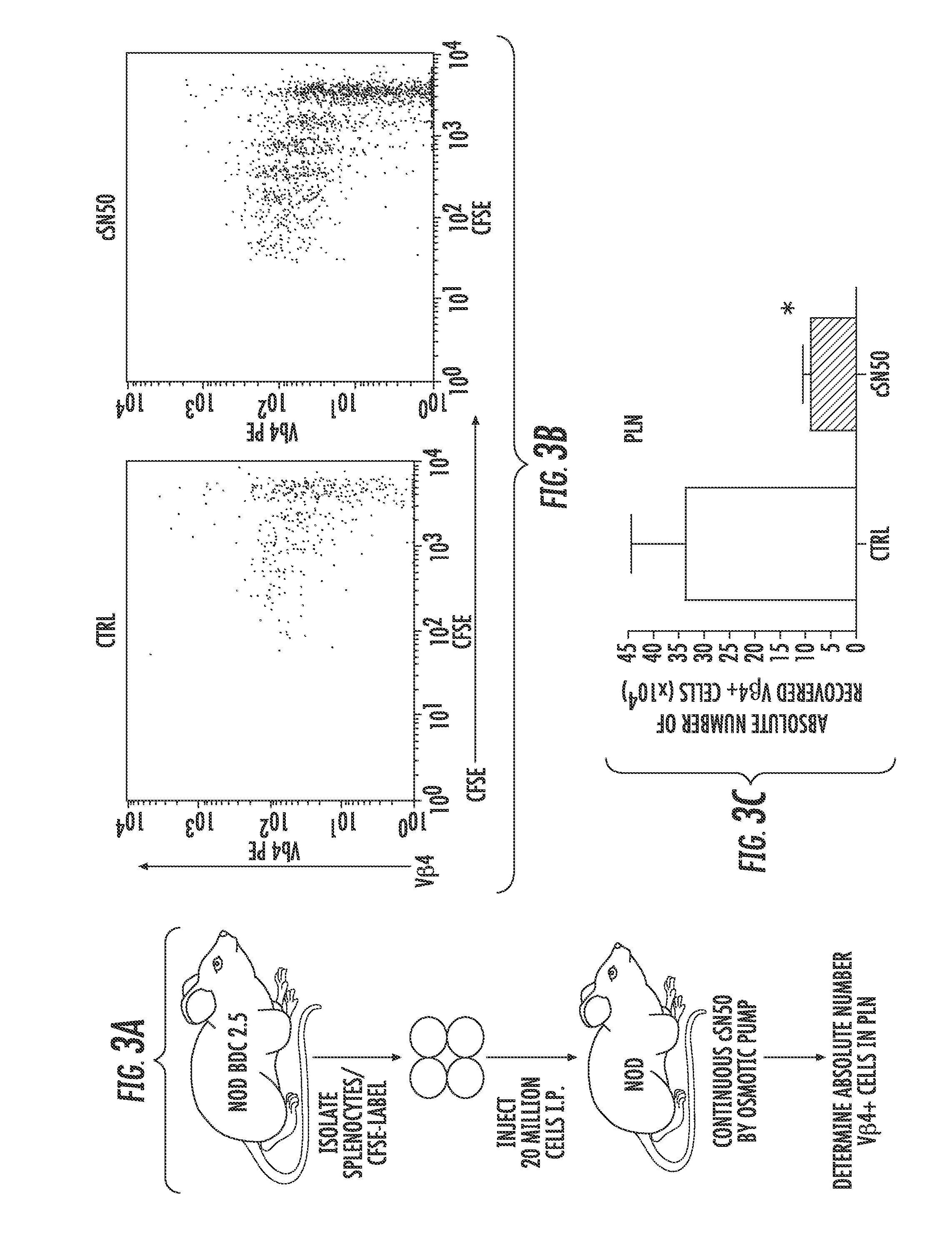
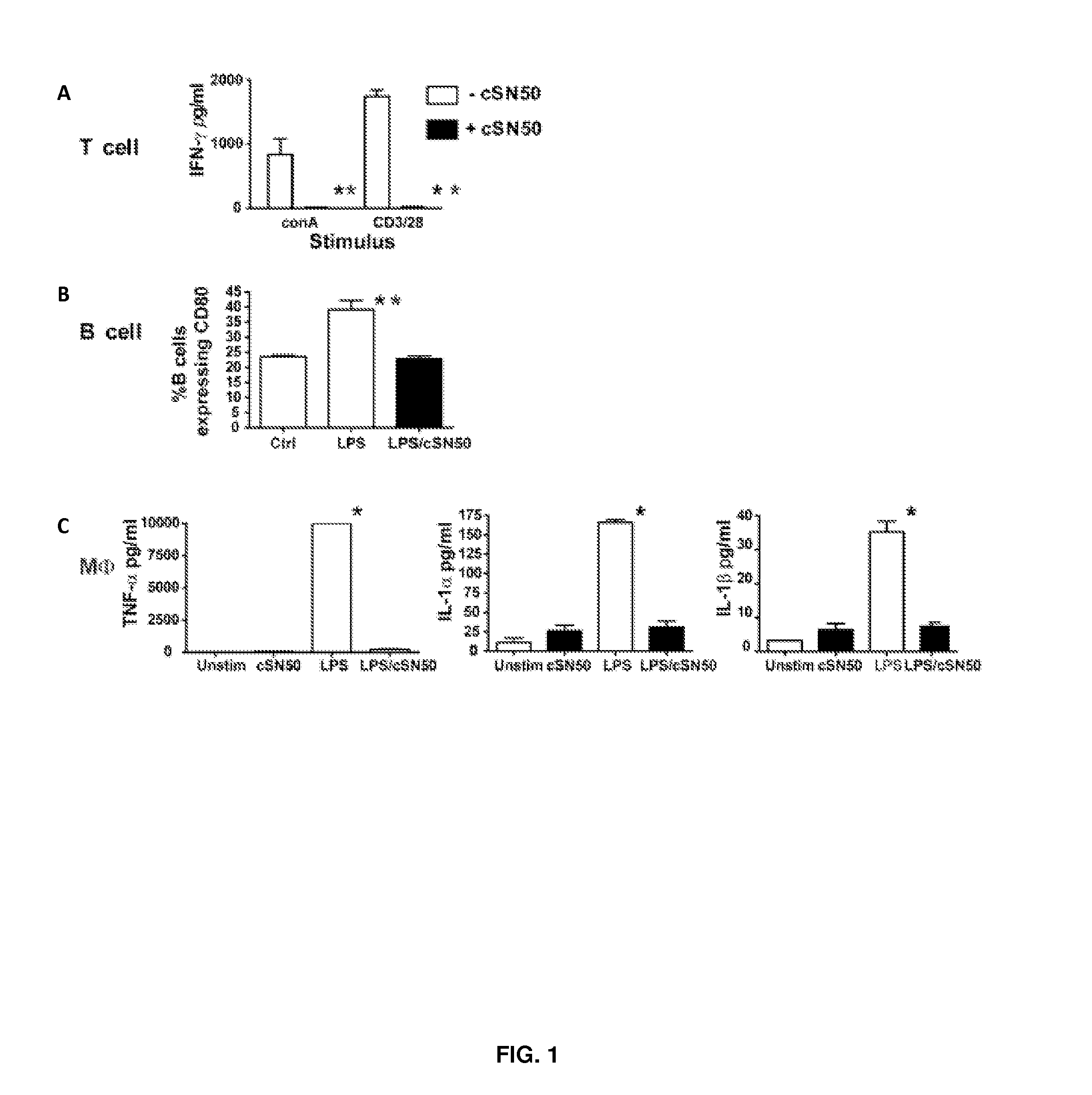
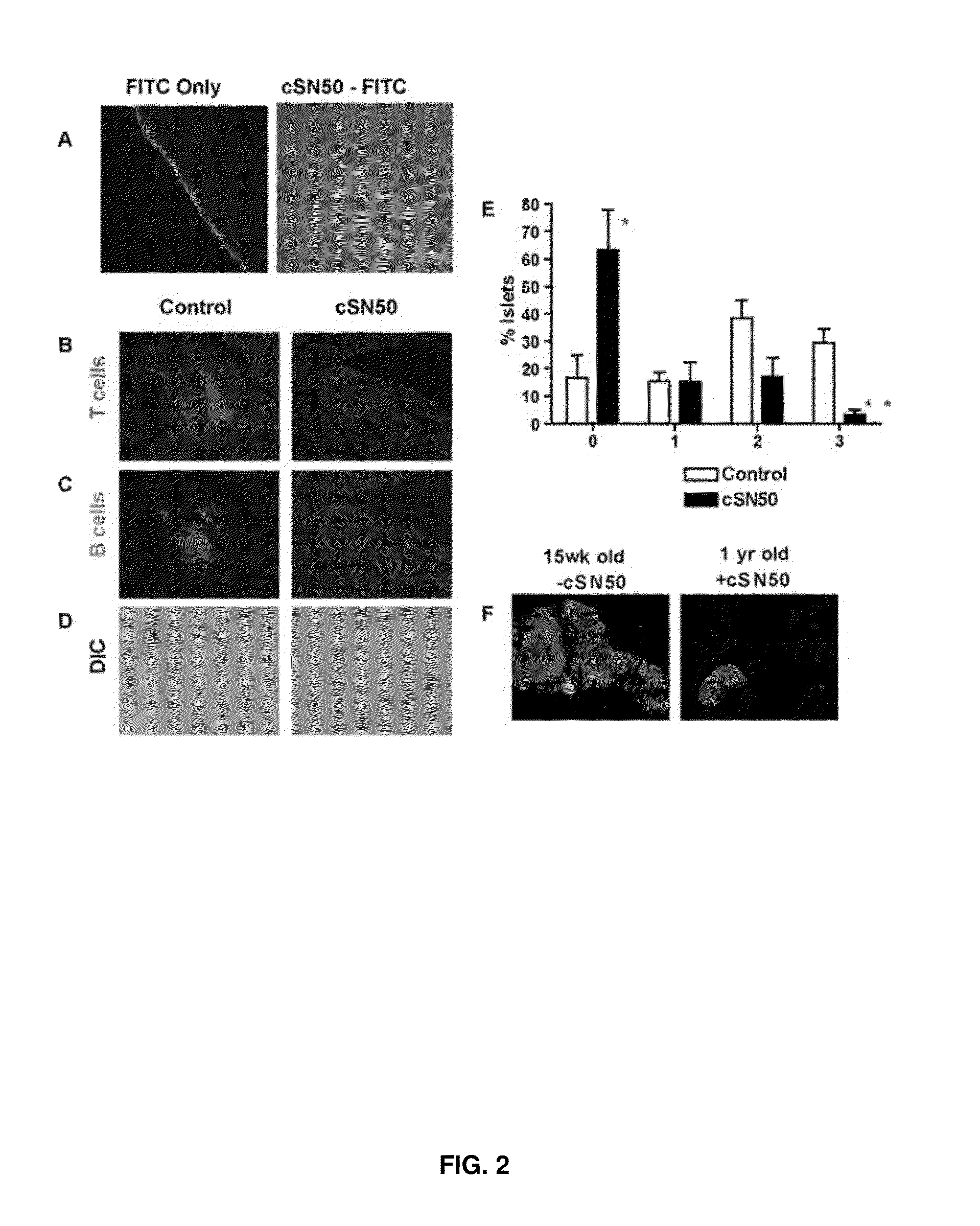
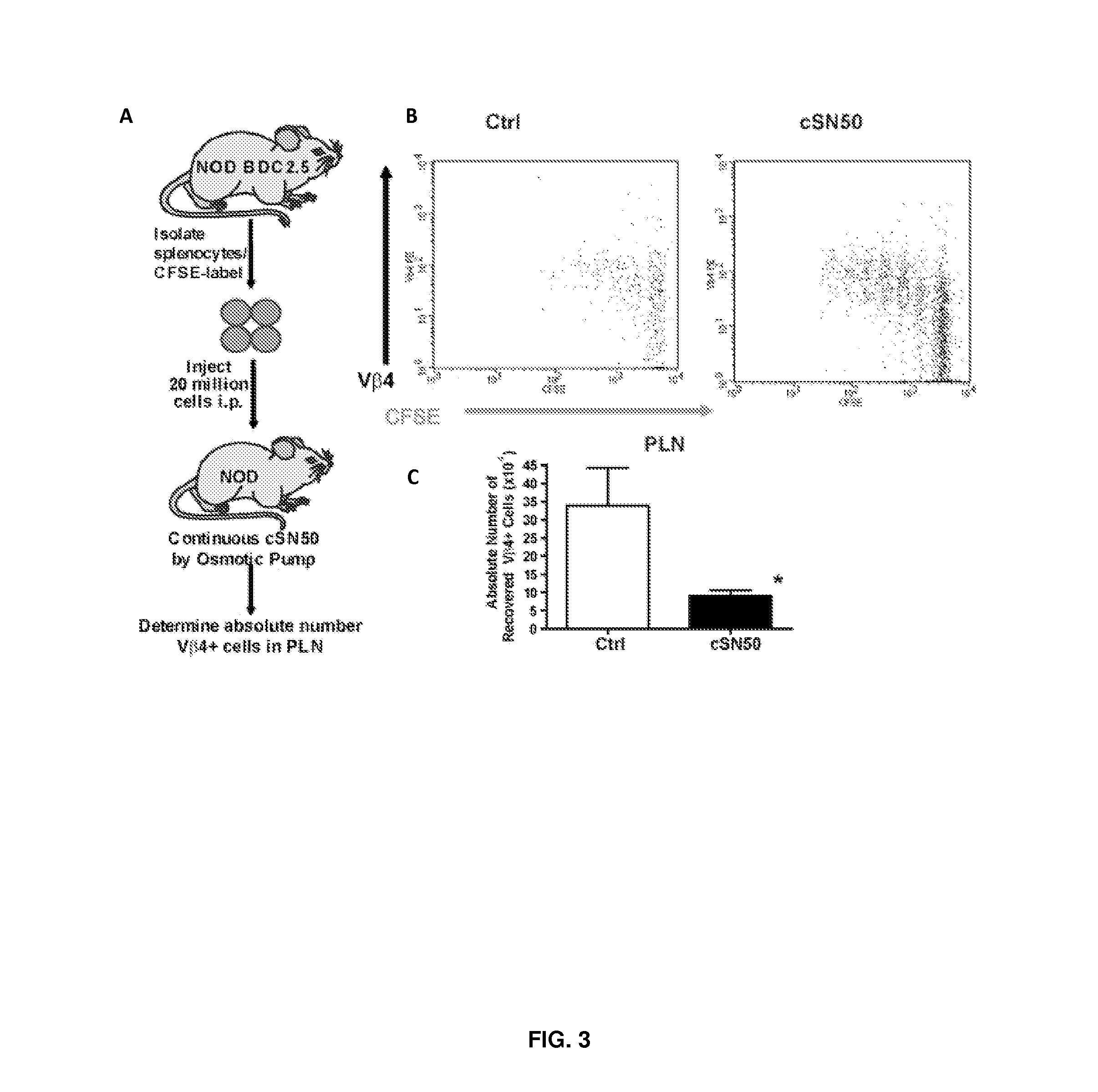
Nuclear Transport Modifiers such as cSN50 and cSN50.1, afford in vivo islet protection following a 2-day course of intense treatment in autoimmune diabetes-prone, non-obese diabetic (NOD) mice, a widely used model of Type 1 diabetes (T1D), which resulted in a diabetes-free state for one year without apparent toxicity and the need to use insulin. cSN50 precipitously reduces the accumulation of islet-destructive autoreactive lymphocytes while enhancing activation-induced cell death of T and B lymphocytes derived from NOD mice. cSN50 attenuated pro-inflammatory cytokine and chemokine production in immune cells in this model of human T1D. cSN50 also provides cytoprotection of beta cells, therefore preserving residual insulin-producing capacity. Because intracellular delivery of a Nuclear Transport Modifier peptide such as cSN50 and cSN50.1 can result in lowering of blood glucose levels and may reducing insulin resistance, the compositions, methods and cells described herein can also be used for treating Type 2 diabetes (T2D).
Owner:VANDERBILT UNIV
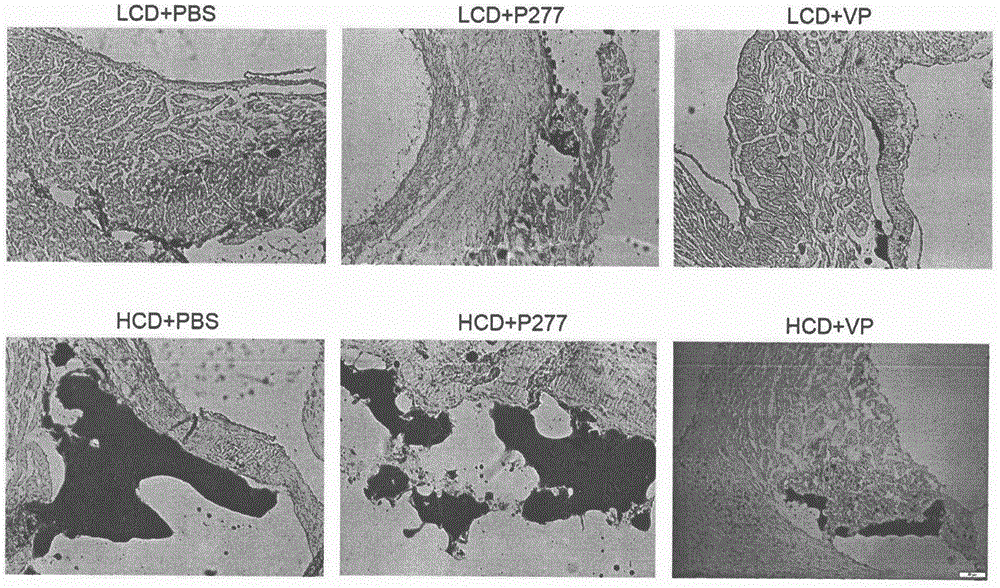
Design and uses of a novel diabetes immunoloregulation peptide-VP peptide
ActiveCN106518987ASlow onsetLevel controlPeptide/protein ingredientsMetabolism disorderSide effectSerum ige
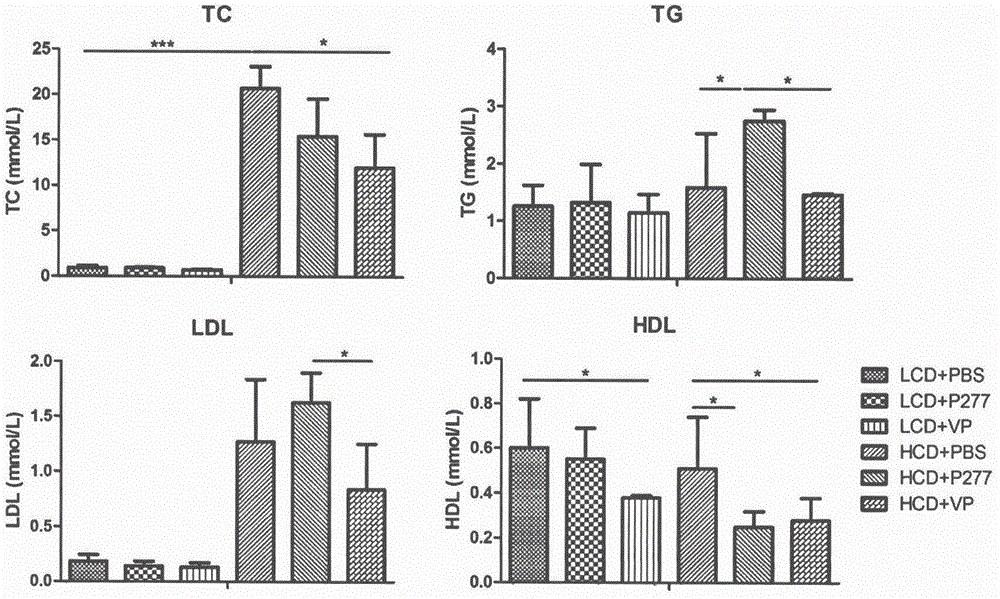
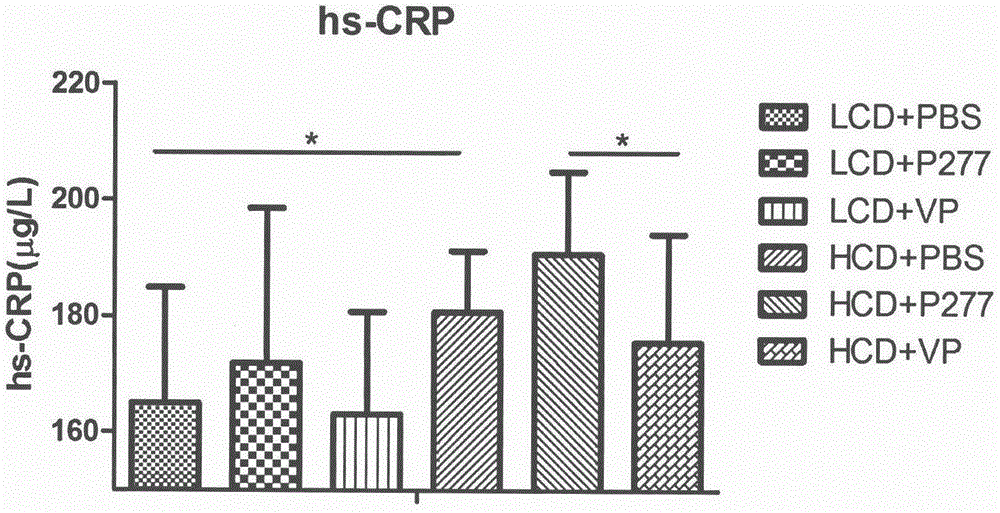
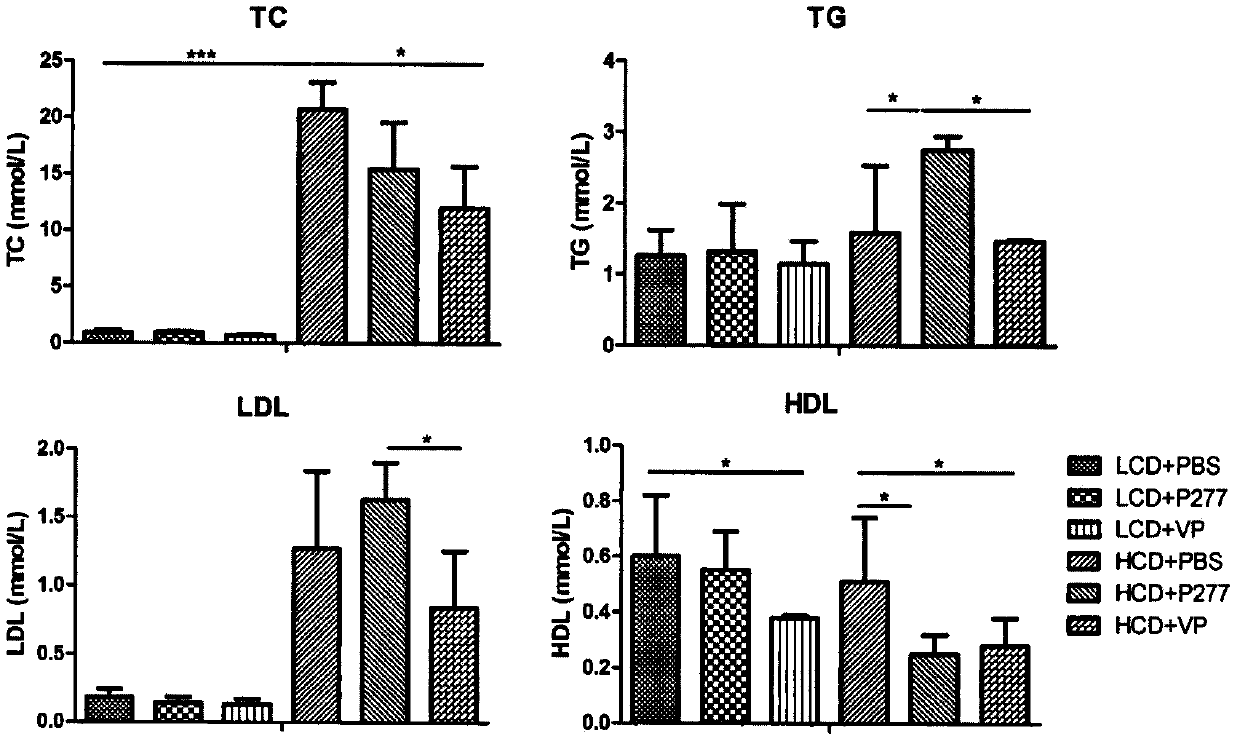
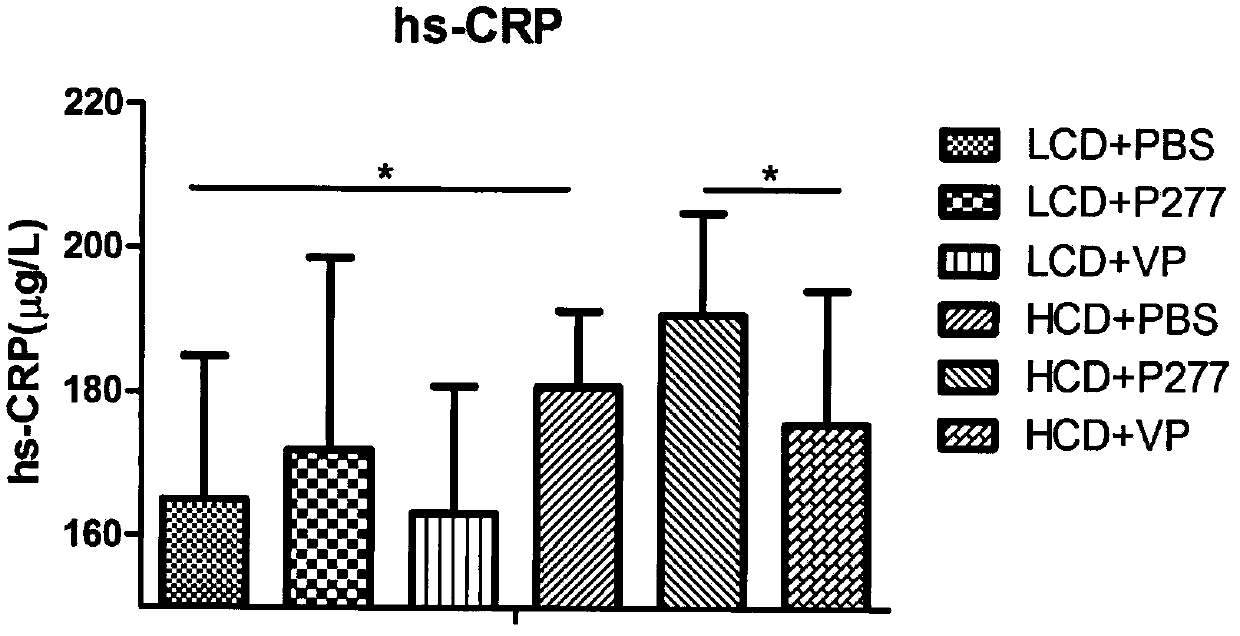
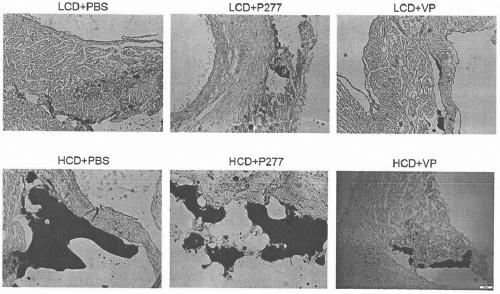
A novel diabetes-related immunoloregulation vaccine peptide VP is designed which can effectively prevent T1DM attack and is free of an atherosclerotic side effect. The VP peptide does not cause lipid disorders in serum of mice, sharp hs-CRP level increases or atherosclerosis side effects. The VP peptide can control blood glucose of NOD mice, maintain normal body weights of mice and reduce the incidence of T1DM. In addition, the VP peptide can maintain normal secretion functions of islet beta cells and can allow the cells to exert normal blood sugar adjusting functions.
Owner:CHINA PHARM UNIV
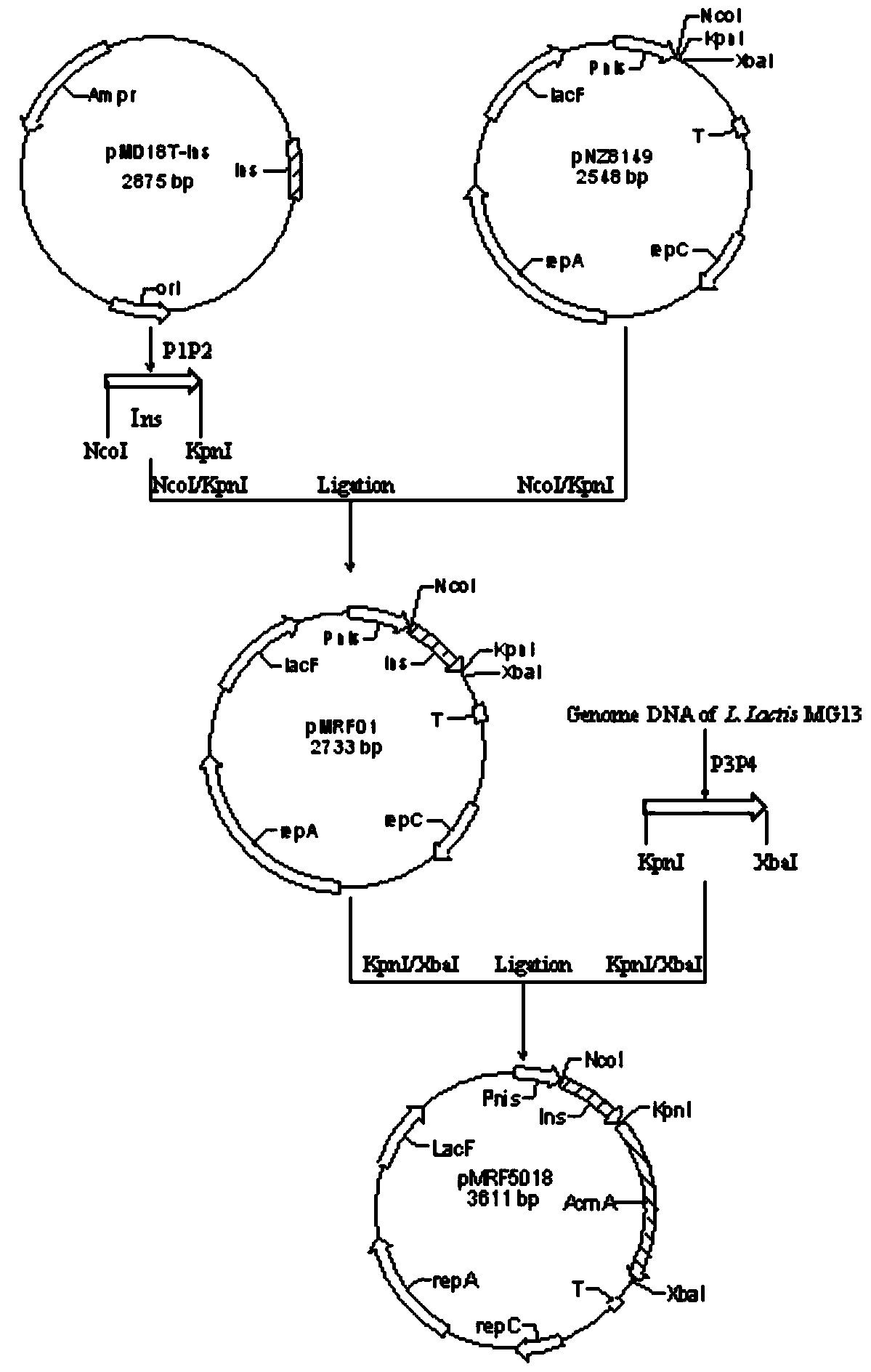
Recombination lactic acid bacteria and application thereof
InactiveCN105255800AImproving immunogenicityHigh titerBacterial antigen ingredientsBacteriaWestern blotIn vivo
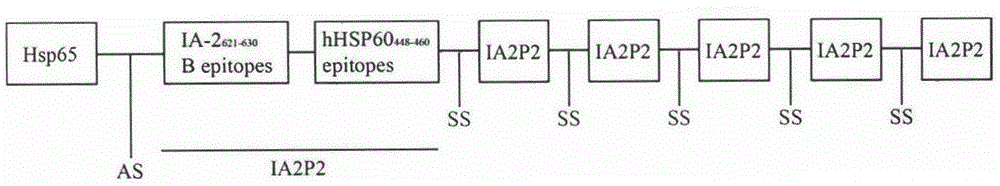
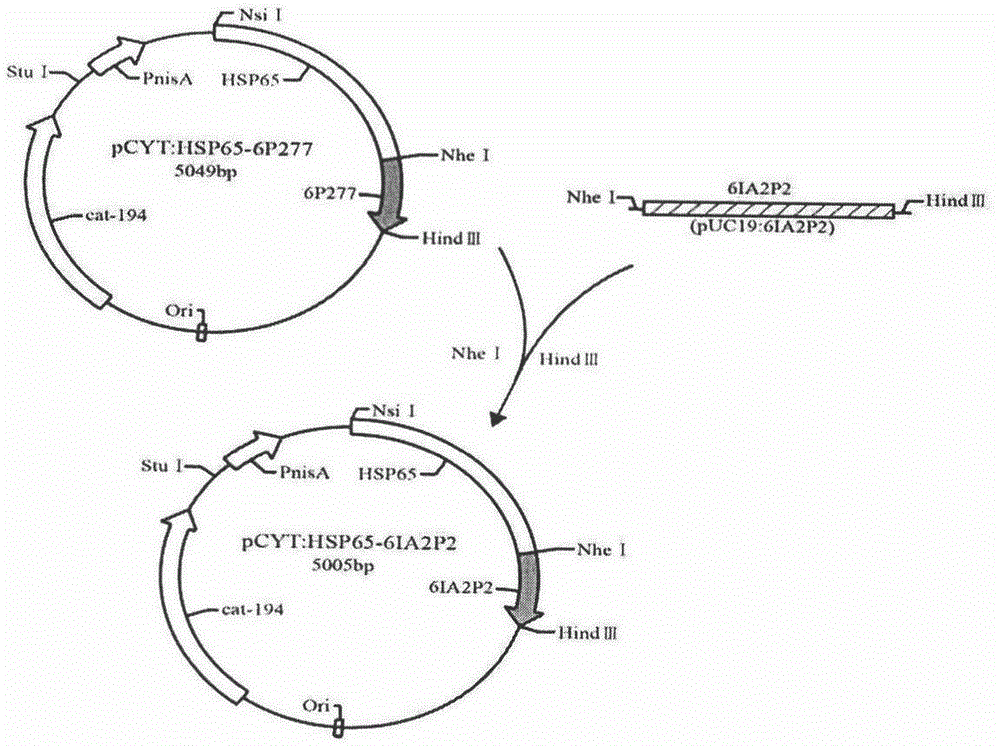
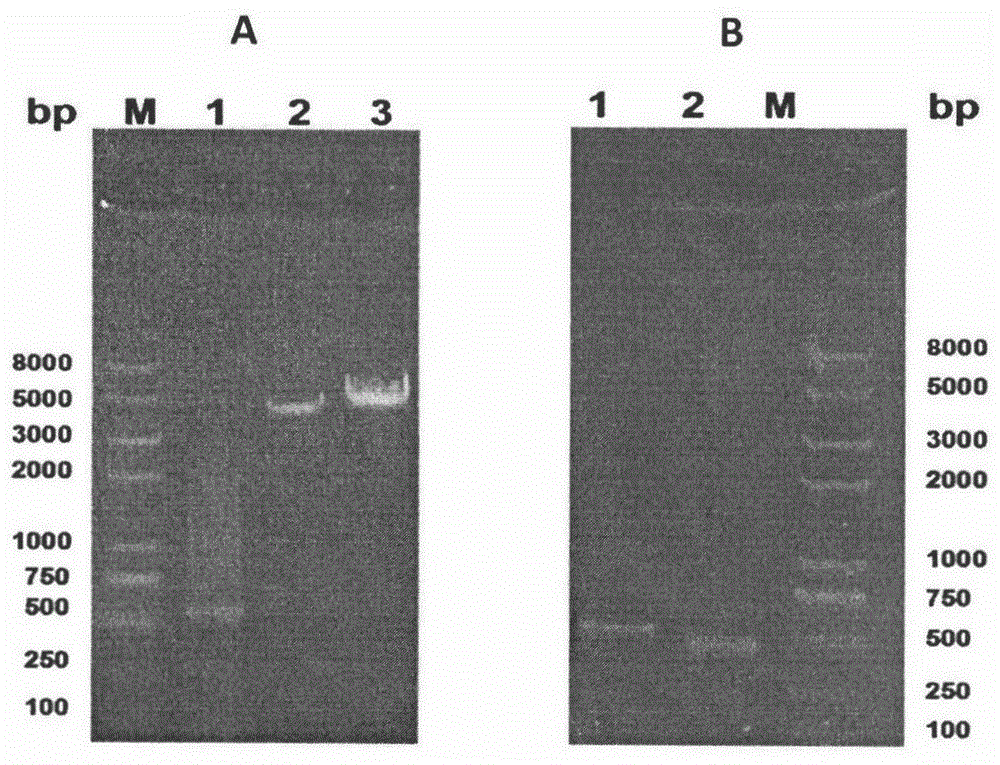
The invention relates to novel I-type diabetes resisting recombination lactic acid bacteria and application thereof, belongs to the field of biological medicine, and discloses an I-type diabetes resisting vaccine with lactococcus lactics as a carrier and a preparing method and application of I-type diabetes resisting vaccine. A micro genetic technology platform of a laboratory is used, the recombination lactococcus lactics vaccine with lactococcus lactics as the carrier, and the recombination lactococcus lactics vaccine expresses fusion protein HSP65-6IA2P2 after induction. The correct expression of the fusion protein vaccine is detected through Western blot authentication. It is proved through in-vivo animal experiments that the vaccine can remarkably reduce the occurrence of NOD mouse diabetes through an oral administration way, can perform the function of controlling I-type diabetes and has good clinical application prospects on preventing diabetes.
Owner:CHINA PHARM UNIV
Delivery of as-oligonucleotide microspheres to induce dendritic cell tolerance for the treatment of autoimmune type 1 diabetes
InactiveUS20100260855A1Avoid accessInduce toleranceOrganic active ingredientsPowder deliveryTolerabilityDendritic cell
AS-oligonucleotides are delivered in microsphere form in order to induce dendritic cell tolerance, particularly in the non-obese-diabetic (NOD) mouse model. The microspheres incorporate antisense (AS) oligonucleotides. A process includes using an antisense approach to prevent an autoimmune diabetes condition in NOD mice in vivo and in situ. The oligonucleotides are targeted to bind to primary transcripts CD40, CD80, CD86 and their combinations.
Owner:UNIVERSITY OF PITTSBURGH +2
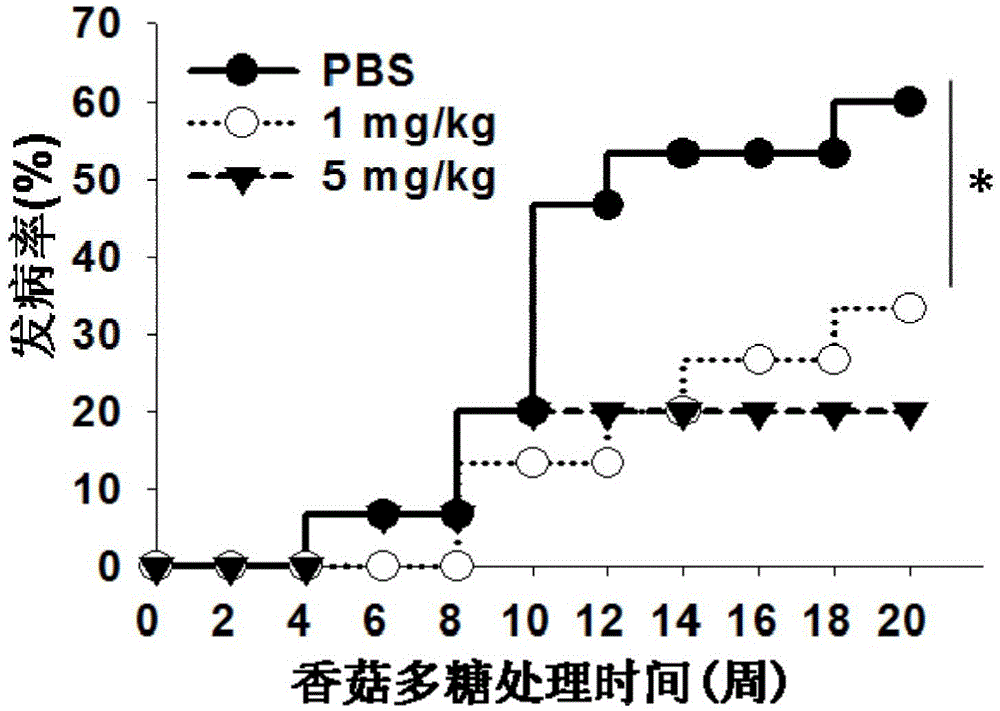
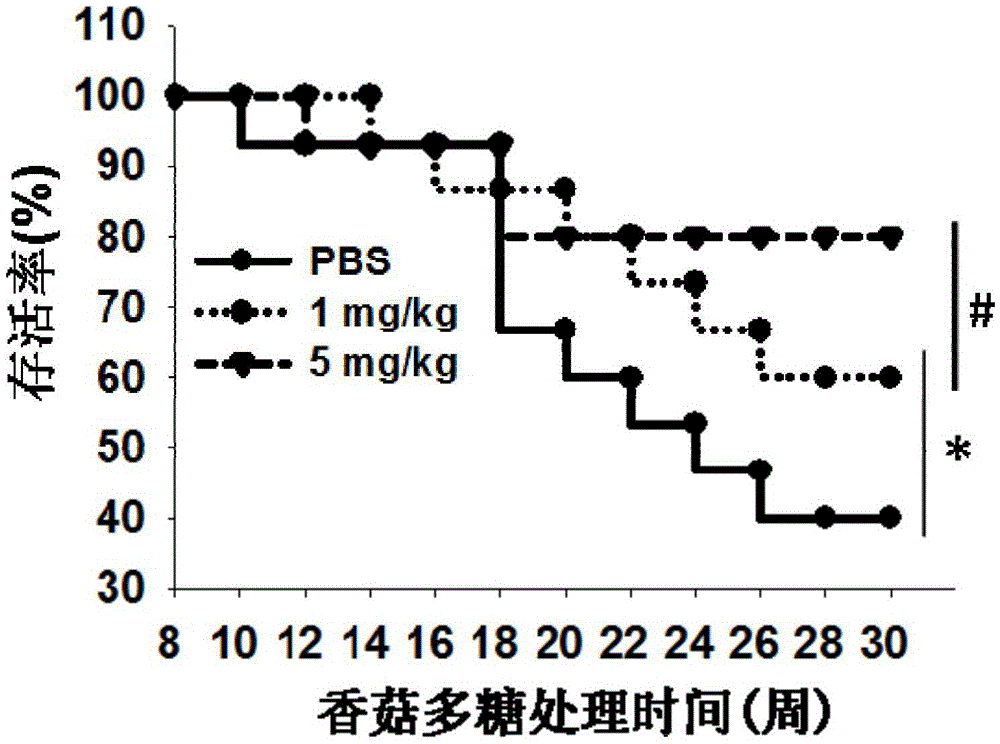
Use of lentinan in preparation of drug for preventing diabetes type 1
InactiveCN105832760AImprove survival rateReduced survival rateOrganic active ingredientsMetabolism disorderCD8T cell
The invention discloses a use of lentinan in preparation of a drug for preventing diabetes type 1. The invention discloses a use of lentinan in preparation of a drug for adjuvant treatment on diabetes type 1. Lentinan is injected into the abdominal cavity of an NOD mouse with spontaneous diabetes type 1 and influence produced by lentinan with body immunity adjustment functions on NOD mouse diabetes incidence is observed. Lentinan reduces NOD mouse insulitis incidence through improving CD25, Foxp3 and T cell (Treg) quantity in NOD mouse spleen, pancreas and lymph nodes, inhibiting CD3, CD8 and cytotoxic T cell (CTL) activation and reducing proinflammatory cytokine secretion, improves a serum insulin level, keeps a blood sugar normal level, finally reduces NOD mouse diabetes type 1 incidence and improves an NOD mouse survival rate.
Owner:NANJING MEDICAL UNIV
Recombination human insulin and its coding gene and application
InactiveCN101148473AConvenient treatmentBacteriaPeptide/protein ingredientsInsulin activityInsulin Gene
The present invention discloses one kind of recombinant human insulin and its coding gene and application. The recombinant human insulin is following protein: 1. the protein comprising the amino acid residue sequence of Sequence 1 in the sequence list; and 2. the derivative protein with the sequence of Sequence 1 through the substitution, deletion and / or addition of amino acid residue(s) and possessing the function of human insulin. The recombinant human insulin has a connecting peptide introduced into the chain A and B sequences of natural insulin to favor to forming beta angle structure, a spatial structure similar to that of natural insulin and insulin activity. The present invention also provides one kind of engineering lactic acid bacteria with the recombinant human insulin gene and capable of surviving in intestinal tract for over 48 hr to stimulate the immune system of NOD mouse to generate specific recombinant human insulin antibody, raise the IL-4 level and induce immunologic tolerance generation.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Compositions for preserving insulin-producing cells and insulin production and treating diabetes
ActiveUS9388224B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsAutoimmune diabetesIn vivo
Nuclear Transport Modifiers such as cSN50 and cSN50.1, afford in vivo islet protection following a 2-day course of intense treatment in autoimmune diabetes-prone, non-obese diabetic (NOD) mice, a widely used model of Type 1 diabetes (T1D), which resulted in a diabetes-free state for one year without apparent toxicity and the need to use insulin. cSN50 precipitously reduces the accumulation of islet-destructive autoreactive lymphocytes while enhancing activation-induced cell death of T and B lymphocytes derived from NOD mice. cSN50 attenuated pro-inflammatory cytokine and chemokine production in immune cells in this model of human T1D. cSN50 also provides cytoprotection of beta cells, therefore preserving residual insulin-producing capacity. Because intracellular delivery of a Nuclear Transport Modifier peptide such as cSN50 and cSN50.1 can result in lowering of fasting blood glucose levels and may ameliorate (e.g., reduce, eliminate) insulin resistance, the compositions, methods and cells described herein can also be used for treating Type 2 diabetes (T2D).
Owner:VANDERBILT UNIV
Alb-uPA-teton lentiviral vector and preparation method and application thereof
InactiveCN106676135AAbility to establish an independent systemNucleic acid vectorFermentationTrans-ActivatorsNod mouse
The invention relates to an Alb-uPA-teton lentiviral vector and a preparation method and application thereof. The Alb-uPA-teton lentiviral vector contains an albumin promoter PAlb and a urokinase type plasminogen activator gene uPA which are reversely connected, wherein the albumin promoter PAlb is used for regulating the expression of a tetracycline trans-activator rtTA, and the urokinase type plasminogen activator gene uPA is regulated by a PTight promoter to express. The Alb-uPA-teton lentiviral vector has the advantages that the ability of establishing a system is realized, and the expression of uPA in an NOD mouse is detected.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
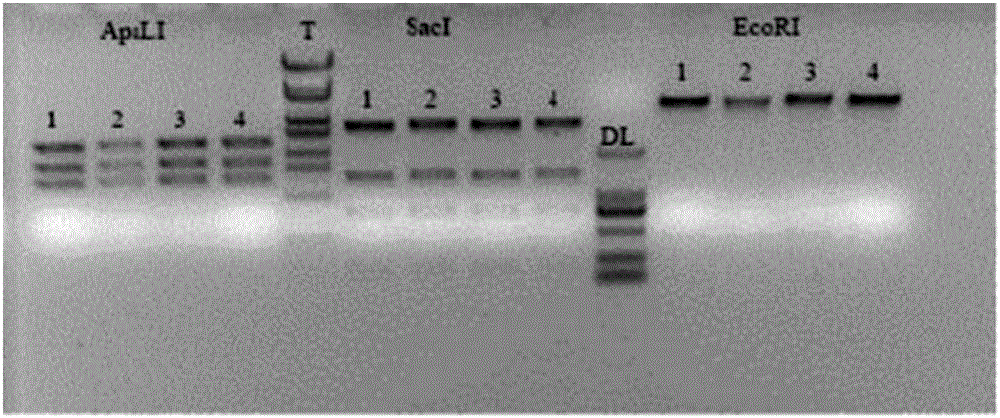
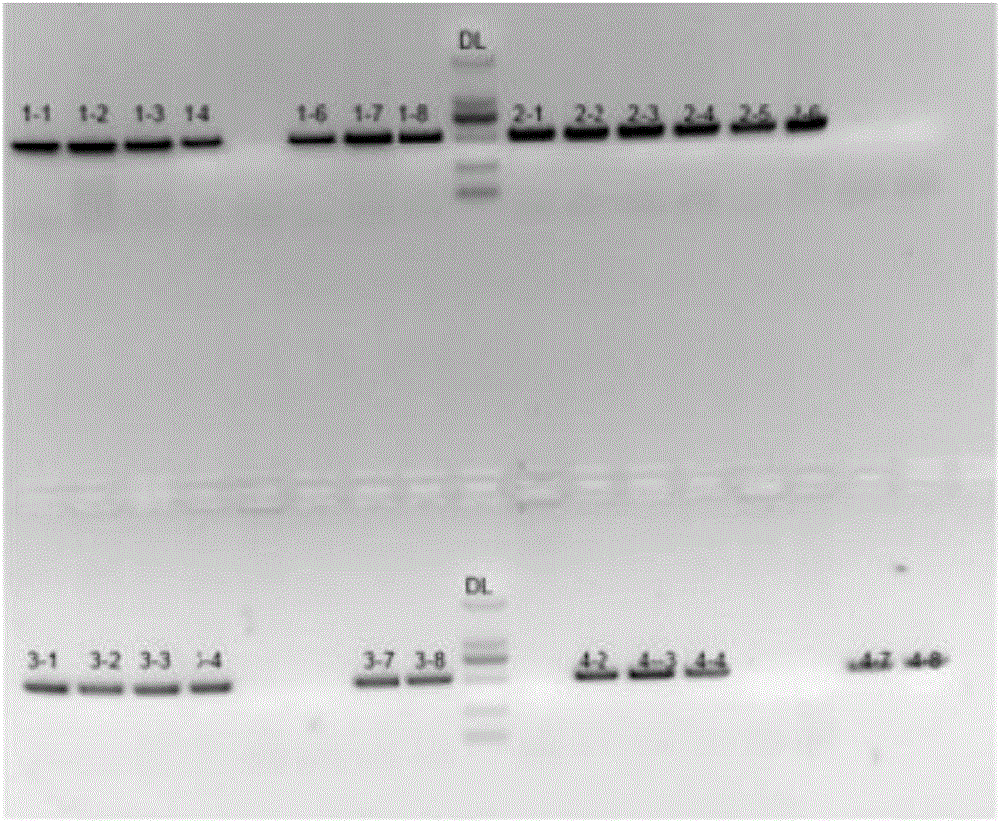
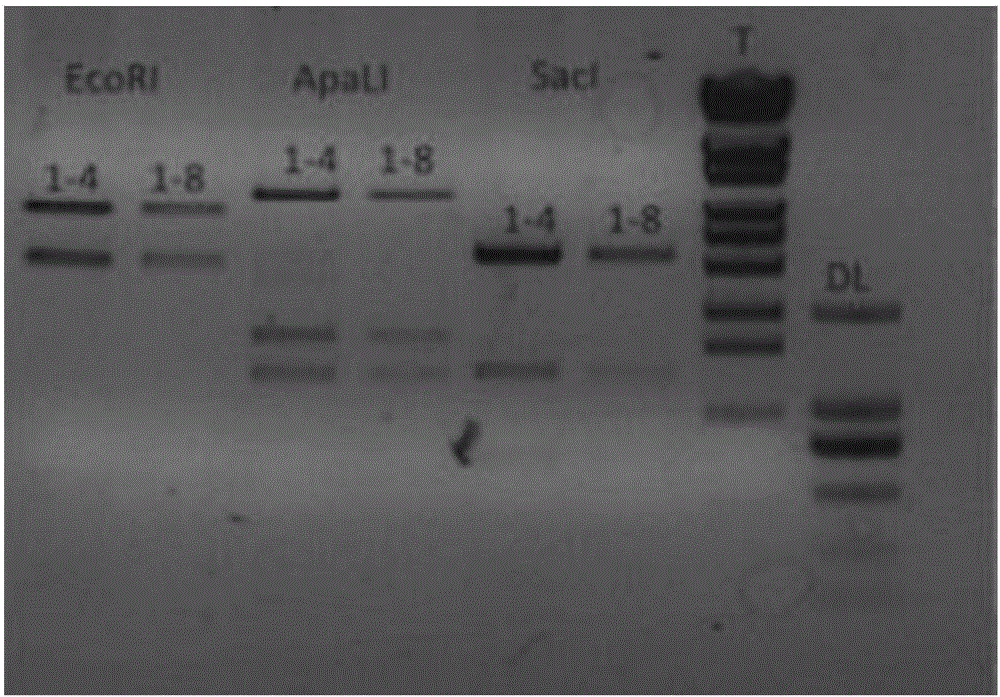
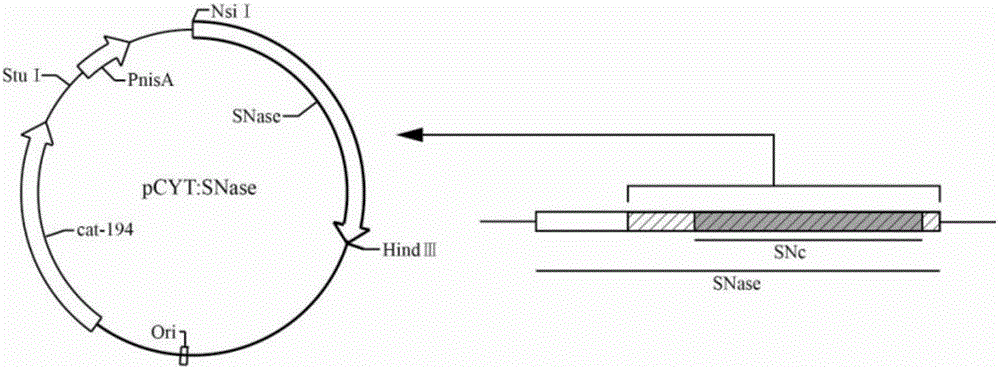
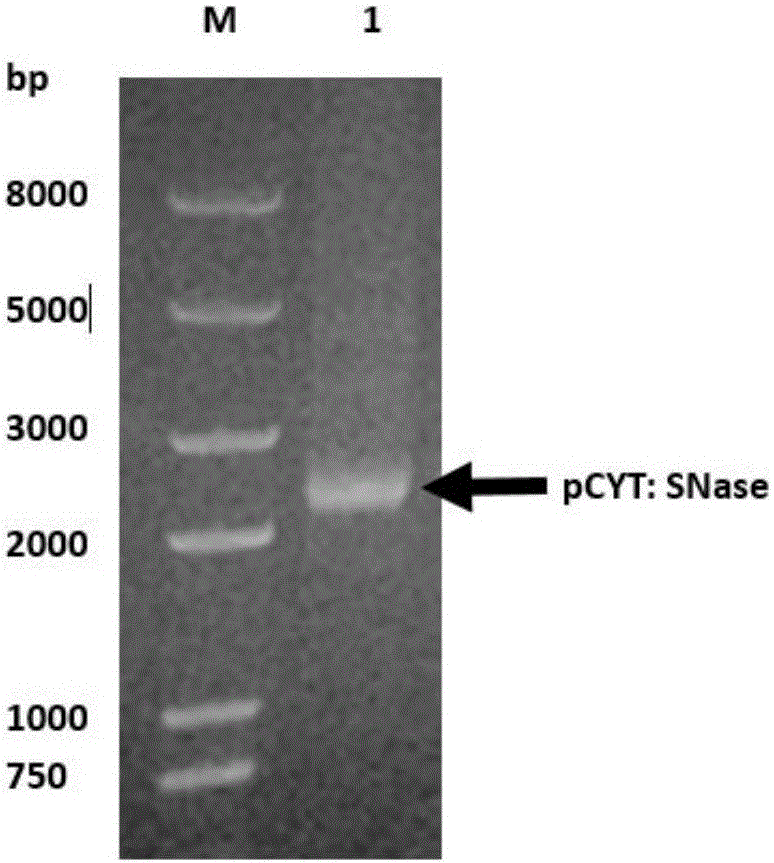
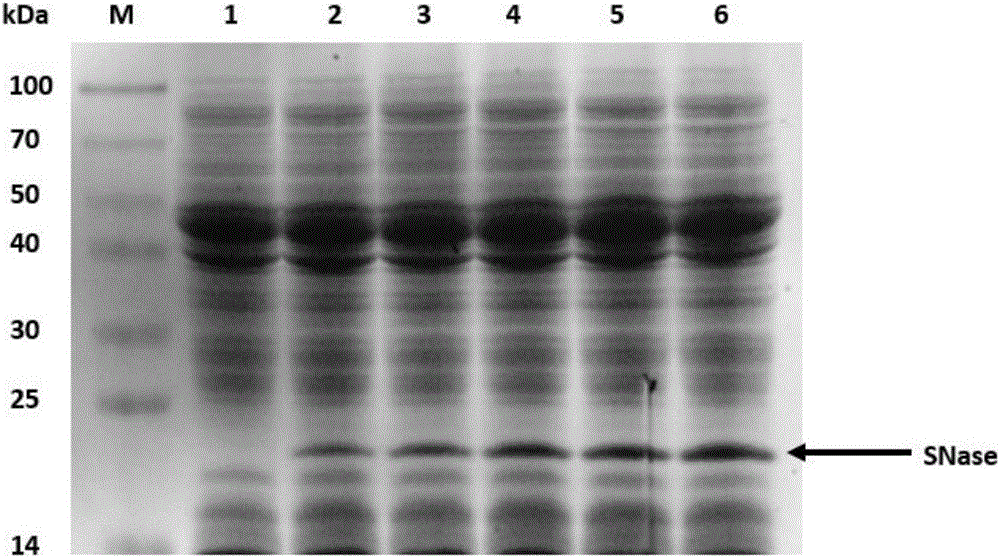
Application of recombinant lactobacillus and SNase in preparing drug for preventing or treating I type diabetes mellitus
ActiveCN105770868AHas hypoglycemic effectAvoid degradationPeptide/protein ingredientsHydrolasesTGE VACCINEStaphylococcal Nuclease
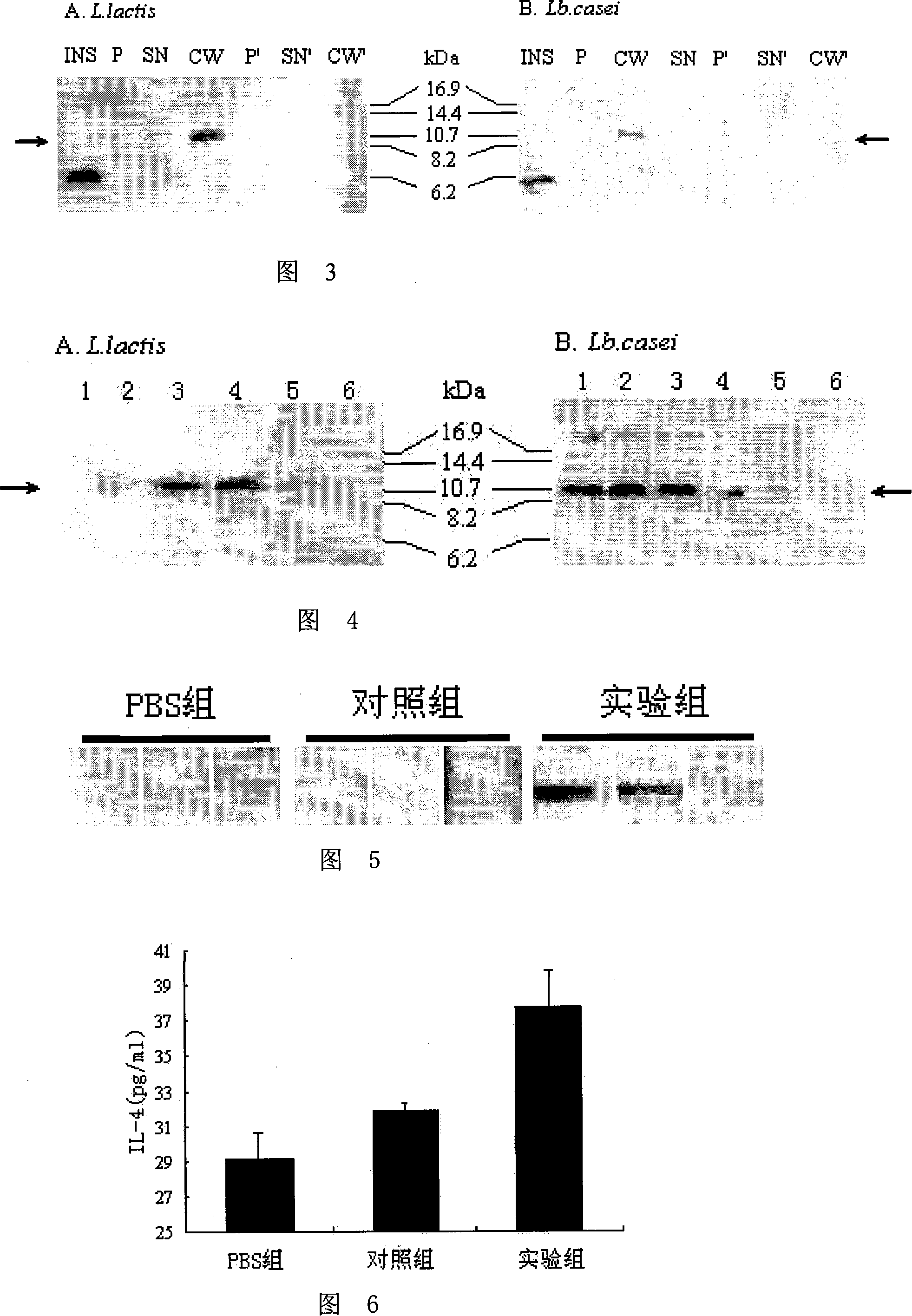
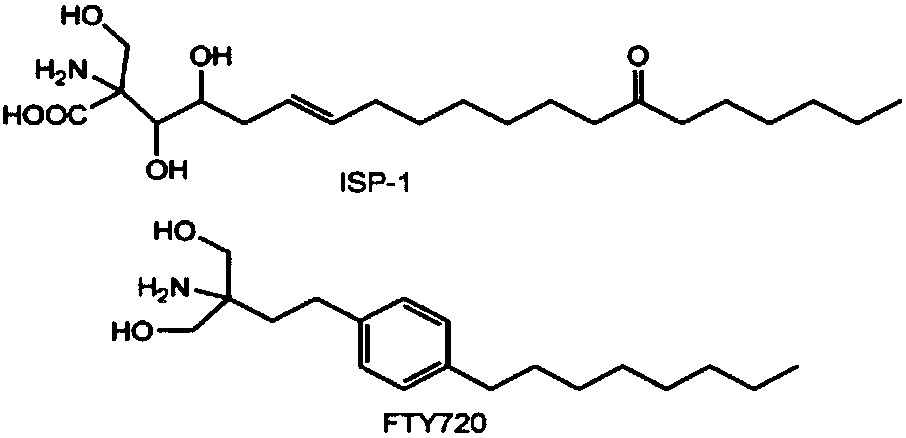
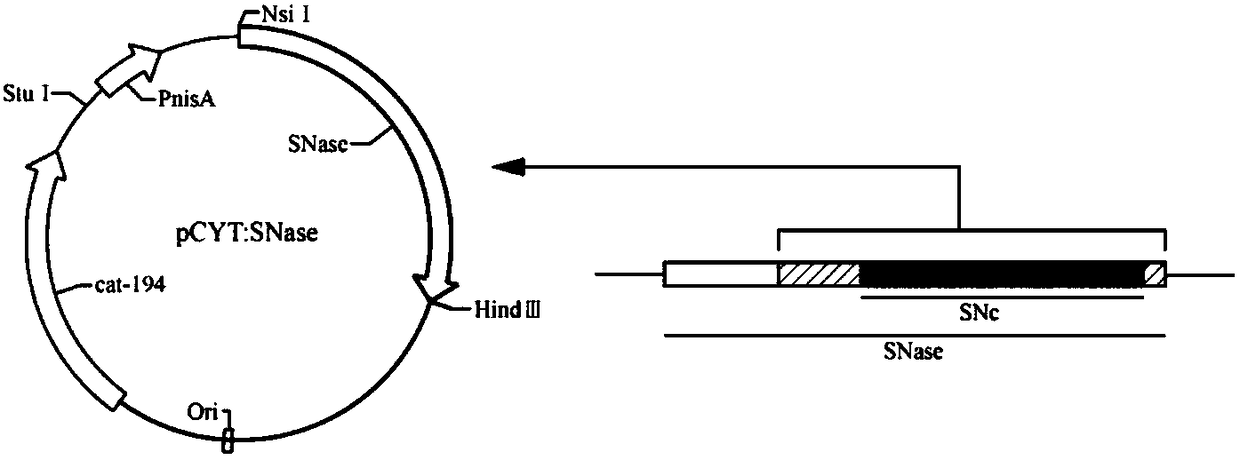
The invention relates to application of recombinant lactobacillus and SNase in preparing a drug for preventing or treating I type diabetes mellitus. The purpose is to provide new application of a vaccine with recombinant lactobacillus L.lactis NZ9000 p CYT: SNase as a carrier in resisting I type diabetes mellitus. The recombinant lactobacillus can express destination protein staphylococcal nuclease, SNase after being induced by lactose. The L.lactis NZ9000 p CYT: SNase living bacterium vaccine is used for immunity of female NOD / LtJ mice four weeks old in an administering oral medication mode, and a series of pharmacodynamic indexes are evaluated, it is found that morbidity of NOD mouse diabetes can be obviously reduced by orally taking L.lactis NZ9000 p CYT: SNase, blood sugar and body weight of the NOD mice can be controlled well, and the survival rate of the NOD mice can be increased remarkably. Therefore, the recombinant lactobacillus L.lactis NZ9000 p CYT: SNase can obviously restrain occurrence and development of NOD mouse diabetes and can be used for developing drugs for I type diabetes mellitus.
Owner:CHINA PHARM UNIV
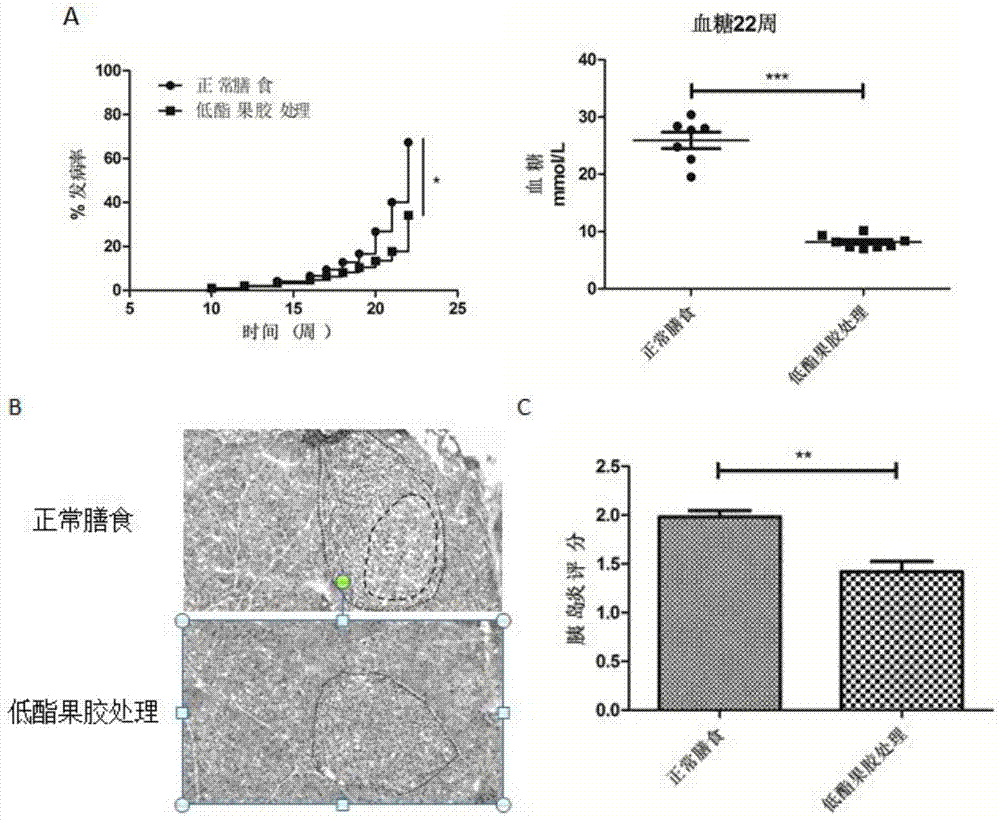
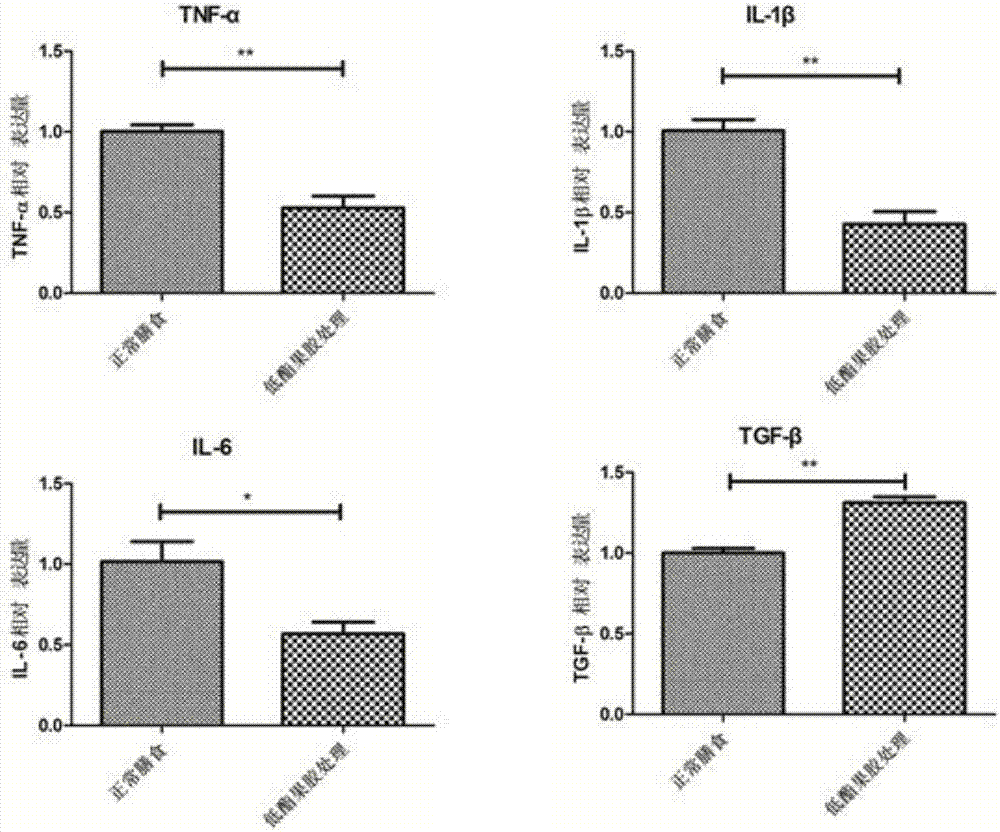
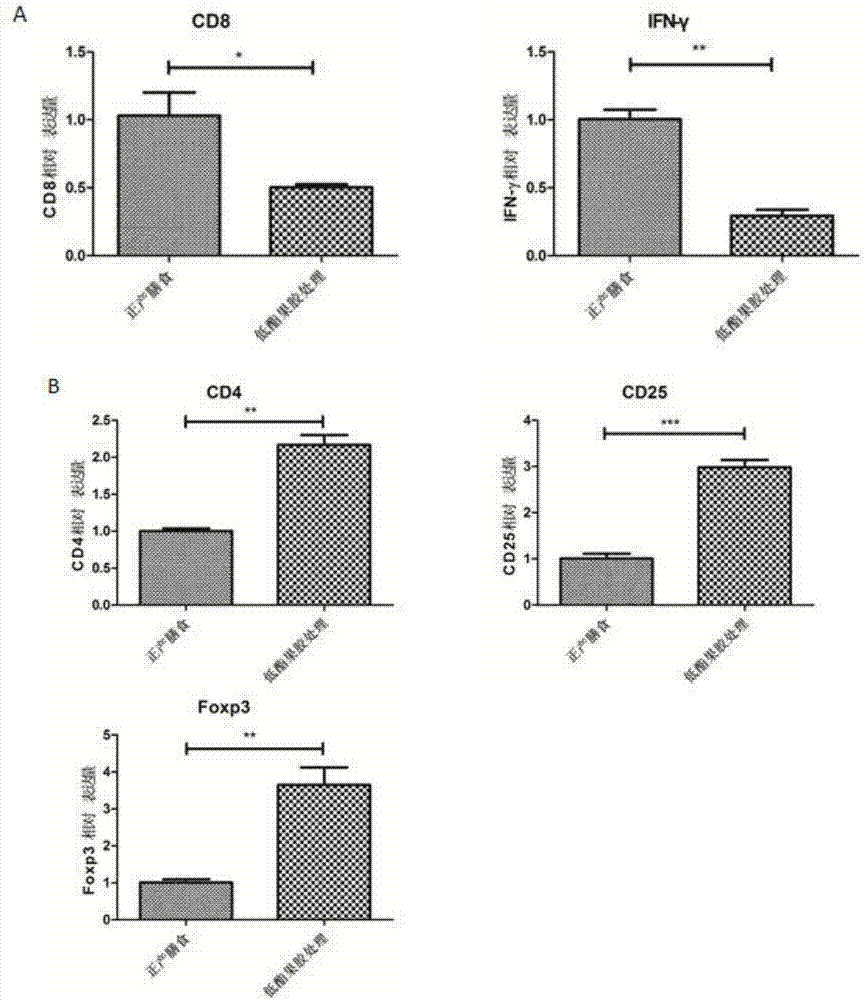
Applications of low methoxyl pectin in preventing and controlling, or auxiliary treatment of diabetes
InactiveCN107158026AImprove protectionExcellent indicatorsOrganic active ingredientsMetabolism disorderSurface markerIslet cells
The invention discloses applications of low methoxyl pectin in preventing and controlling, or auxiliary treatment of diabetes, and belongs to the technical field of prevention and treatment of type 1 diabetes mellitus. The invention provides effects of low methoxyl pectin on diabetes caused by high blood sugar, and especially effect of low methoxyl pectin in inhibition development and inflammation degree of type 1 diabetes mellitus in clinical treatment. Low methoxyl pectin is used for prevention treatment of non-obese diabetic NOD mice, and pathological tissue morphology observation on pancreatic tissues and islet cells, and detection on mouse pancreas proinflammatory factors, anti-inflammatory cytokine, immunosuppressive T cells, and diabetes-inducing T cell surface markers are carried out. The levels of a plurality of indexes of a low methoxyl pectin group with a normal diet group are compared, it is found that the indexes of the low methoxyl pectin group are better than that of the normal diet group.
Owner:JIANGNAN UNIV
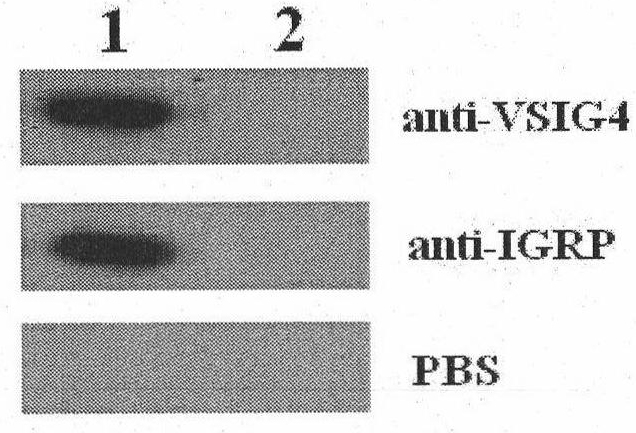
VSIG4 (V-set and Ig domain containing 4) and IGRP (glucose-6-phosphatase catalytic subunit-related protein) dual-gene coexpression recombinant adenovirus as well as preparation method and application thereof
InactiveCN101818133AAvoid damageReduced proliferative capacityMetabolism disorderGenetic material ingredientsT cellIn vivo
The invention discloses a VSIG4 (V-set and Ig domain containing 4) and IGRP (glucose-6-phosphatase catalytic subunit-related protein) dual-gene coexpression recombinant adenovirus as well as a preparation method and an application thereof. The recombinant adenovirus genome contains a VSIG4 and IGRP dual-gene expression cassette which sequentially comprises a CMV (cytomegalovirus) promoter, a VSIG4 full length coding gene, a terminator, an internal ribosome entry site (IRES), an IGRP full length coding gene and a terminator from the upstream to the downstream. The preparation method of the recombinant adenovirus is simple. Dendritic cells of the recombinant adenovirus, which are transfected in vitro, are fed back in vivo, which can reduce the multiplication capacity of lymphocytes of NOD (Nonobese Diabetic) mice with diabete liability and the secretion capacity of cell factors, moreover, the onset time of the diabetes of the NOD mice is delayed, and the morbidity is reduced. The recombinant adenovirus is proved to be capable of effectively inducing the tolerance of specific T-cells and effectively inhibiting the breakage of pancreas islet beta cells and can be used for preparing dendritic cell vaccines for resisting type-1 diabetes.
Owner:ARMY MEDICAL UNIV
Recombinant protein and recombinant gene capable of preventing and curing I type diabetes mellitus through immunity
The present invention provides one kind of restructuring protein and its restructuring gene for preventing and treating type-1 diabetes. Connecting heat shock protein HSP60 originated Mycobacterium bovine to a piece of antigen epitope P277 from human HSP60; restructuring protein produced can be used for immunity directly with no adjuvant. The restructuring protein can stimulate body to produce antigen for P277 both by ways of injection and mucous membrane immunity, decreases marked NOD mouse diabetes occur, can contribute to preventing and caring type-1 diabetes. Meantime, the restructuring gene produced in this invention can also be used to transgene animals or plants and restructuring microorganism as the factory for producing restructuring protein or preparing oral vaccine.
Owner:CHINA PHARM UNIV
Non-obese diabetes mice and its applications
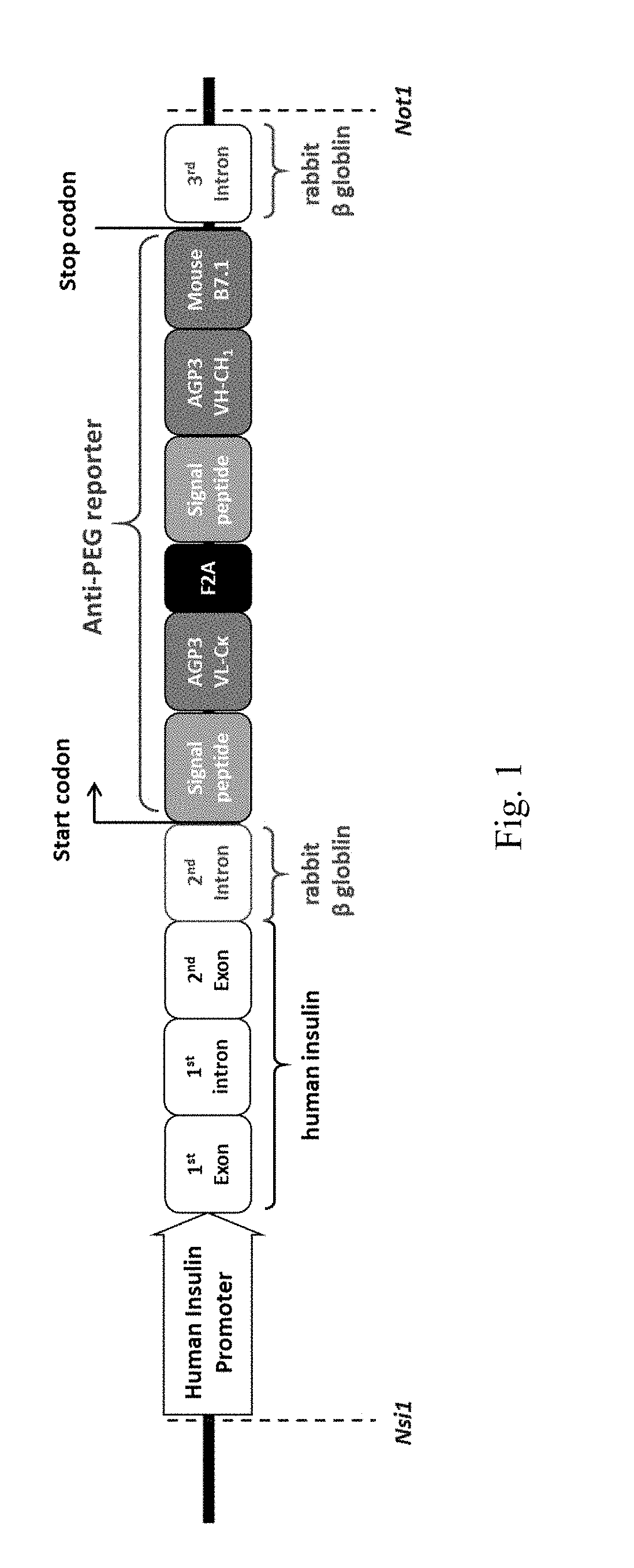
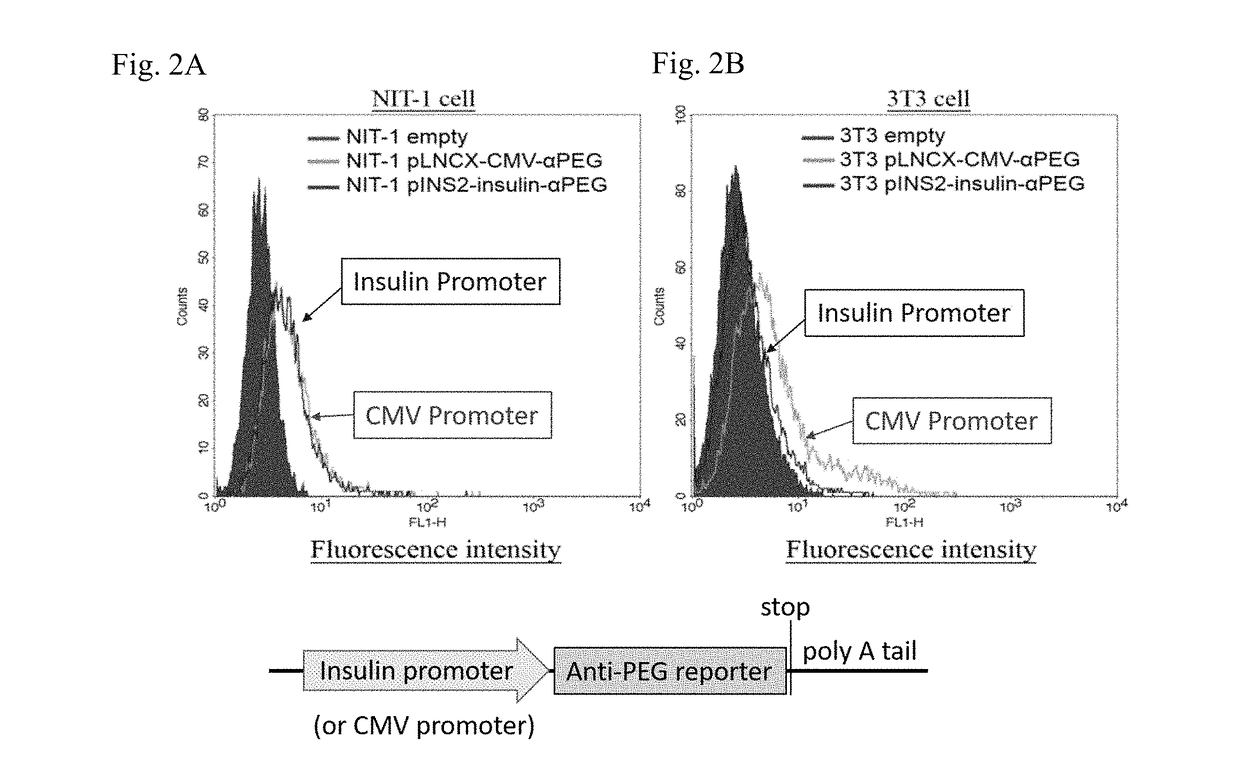
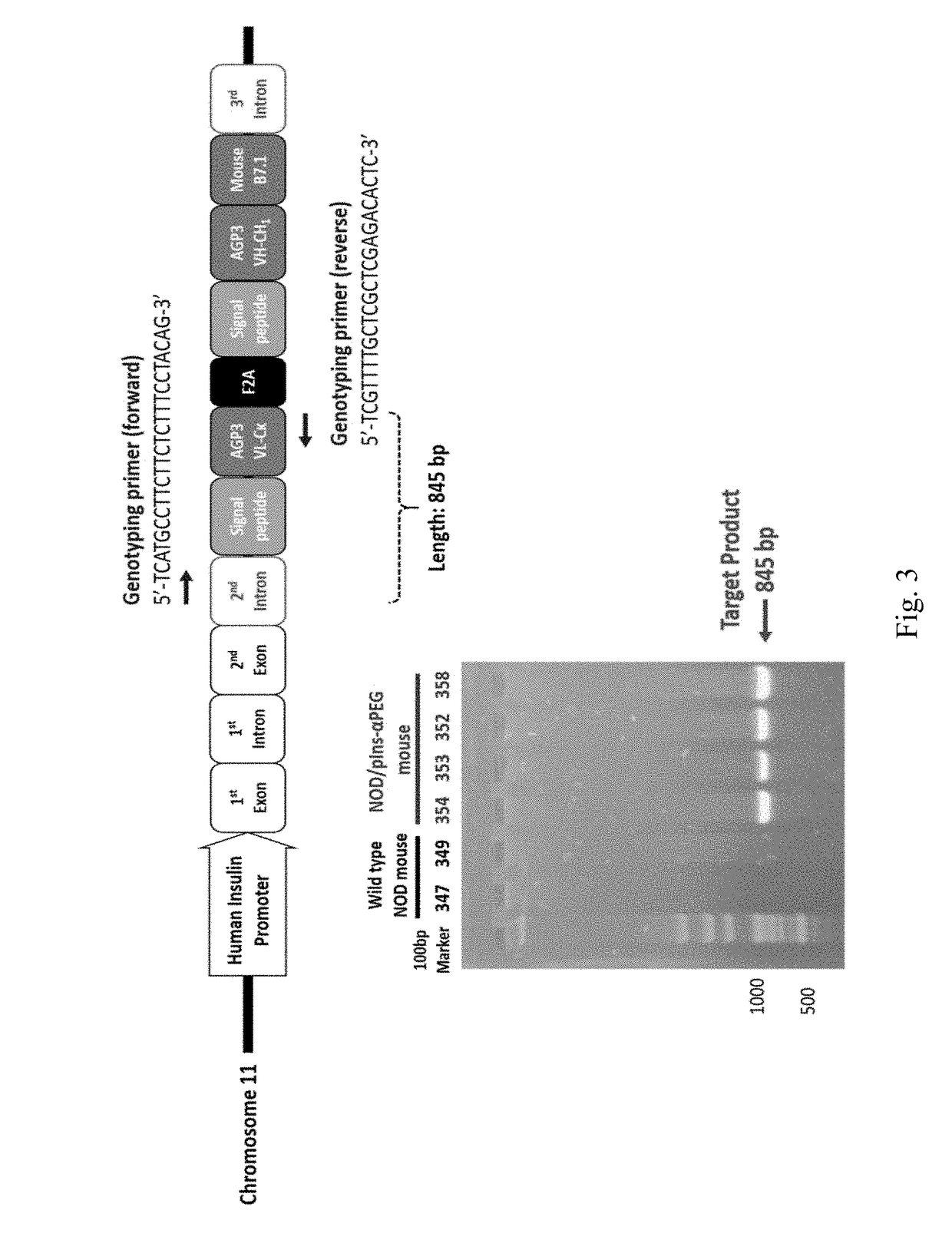
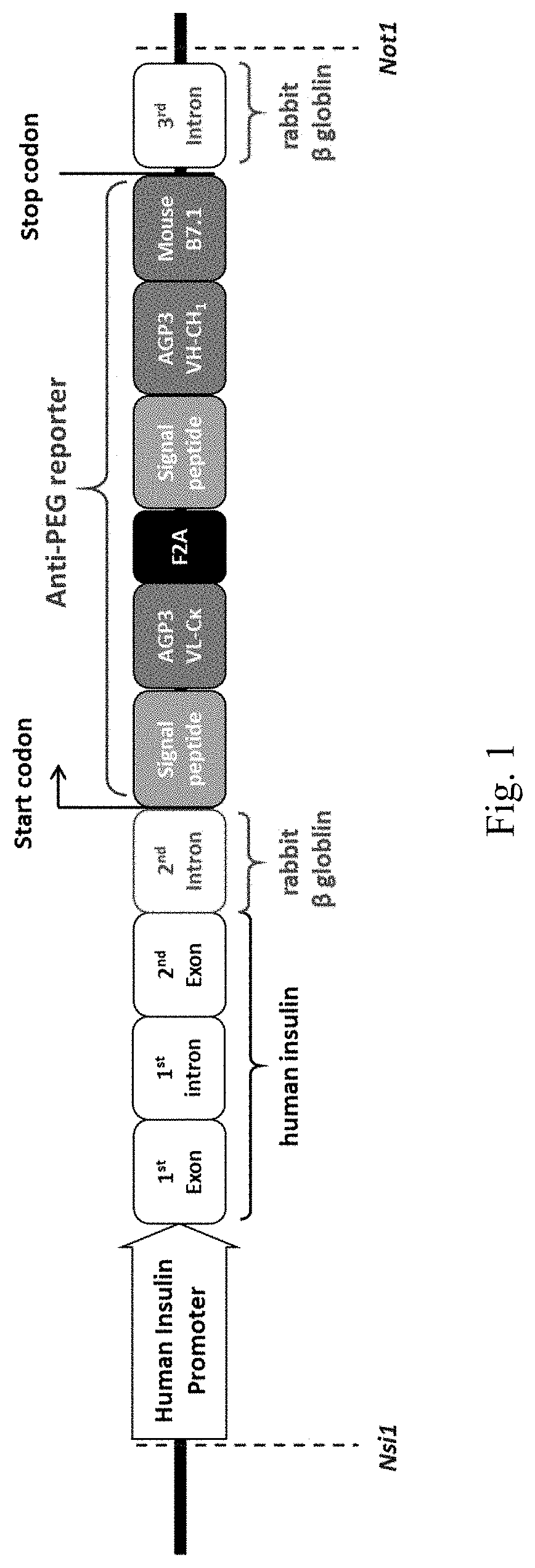
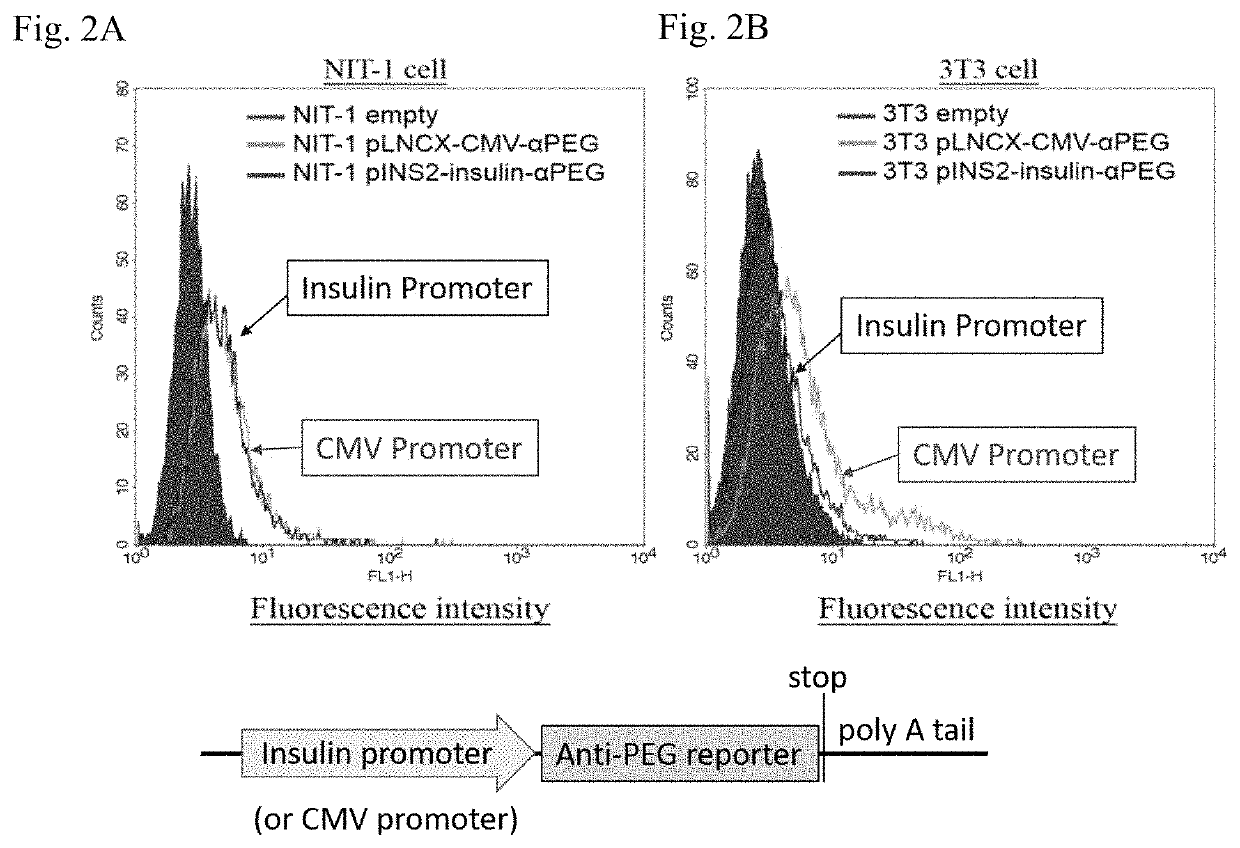
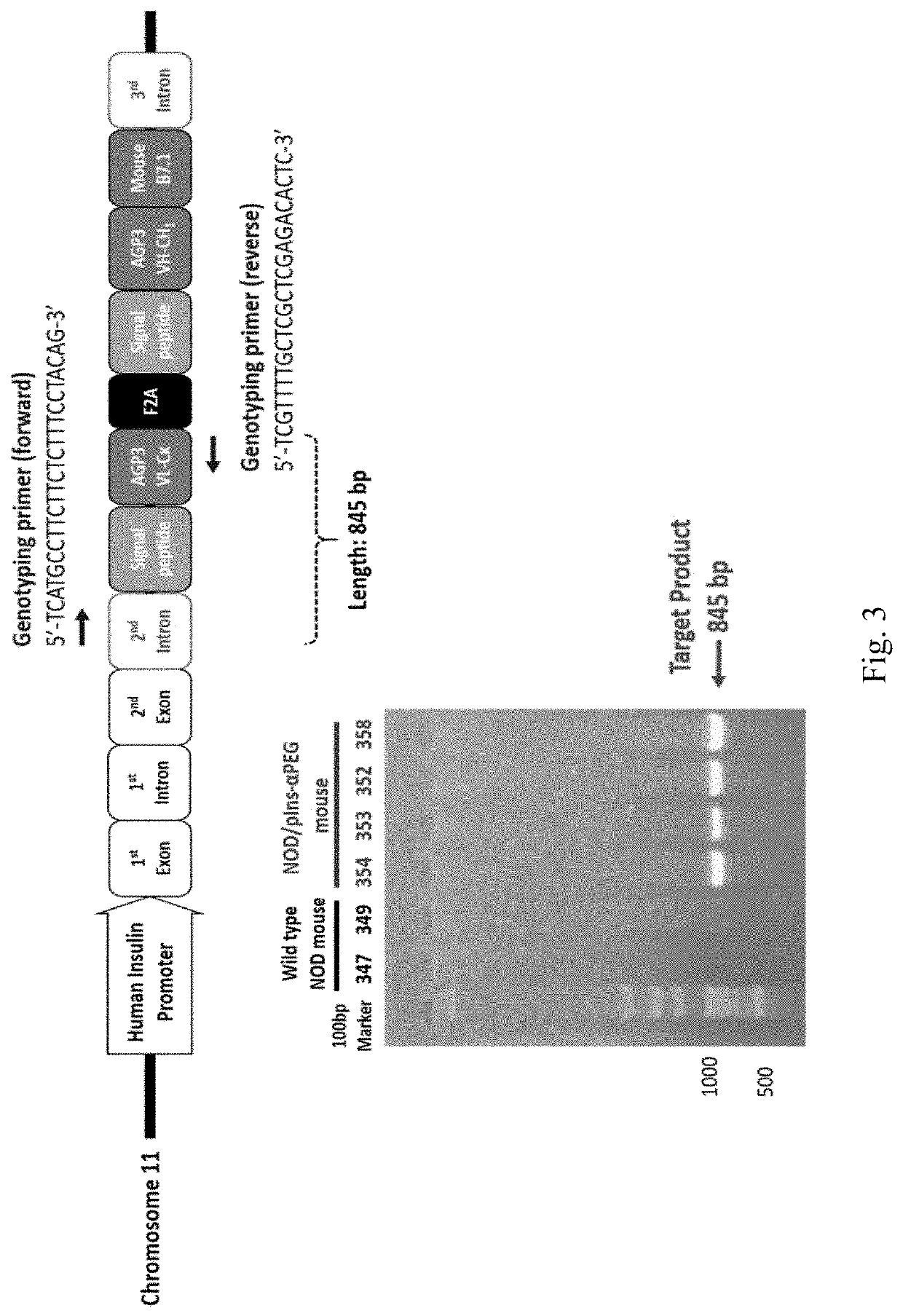
ActiveUS20180146649A1Stable expressionConveniently and accurately tracingImmunoglobulinsBiological testingNon obeseNod mouse
The invention relates to a novel non-obese diabetic (NOD) mouse and its application. Particularly, the invention relates to a NOD mouse specifically expressing anti-polyethylene glycol membrane antibody reporter (anti-PEG reporter) in the NOD mouse.
Owner:TAIPEI MEDICAL UNIV
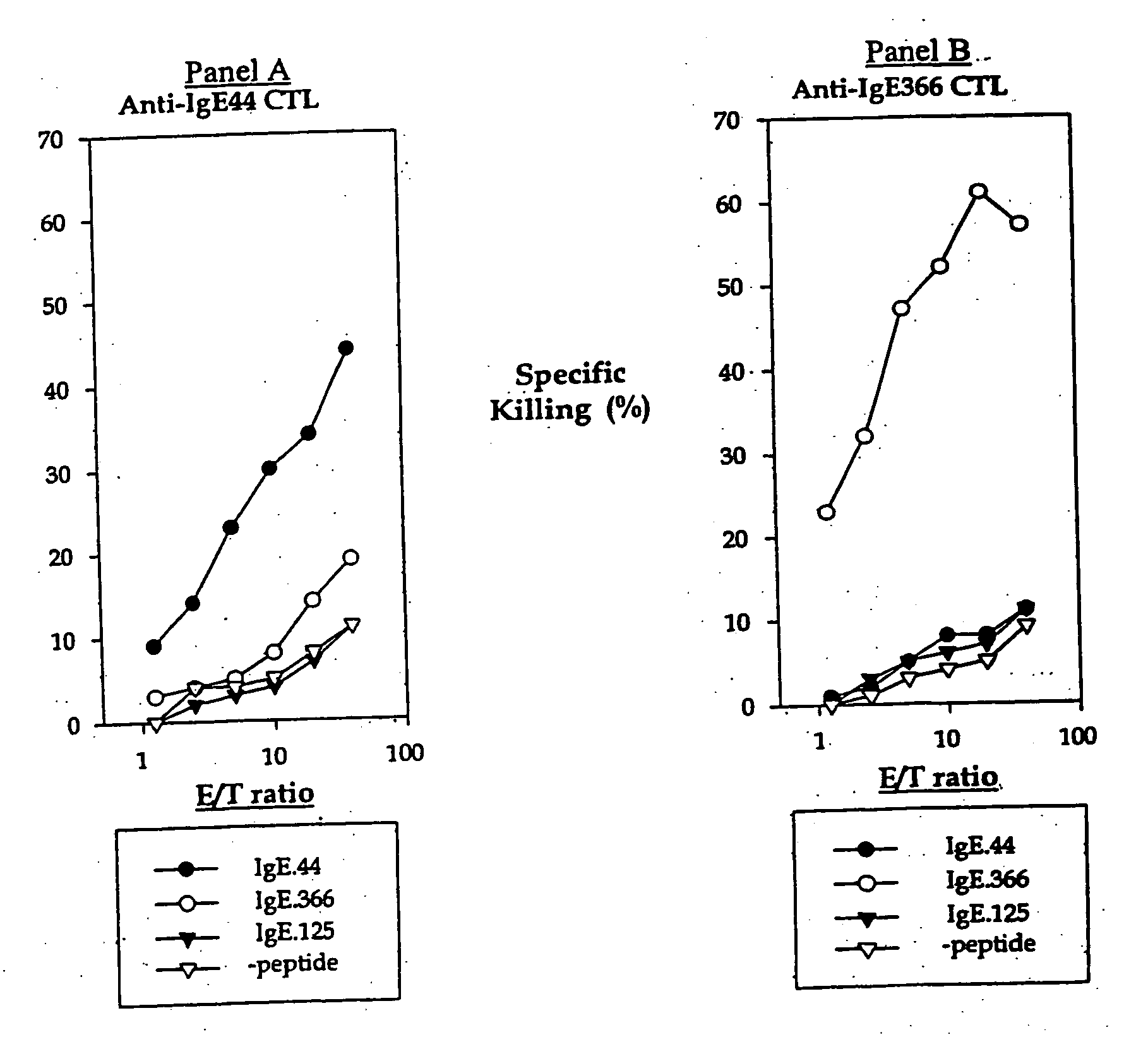
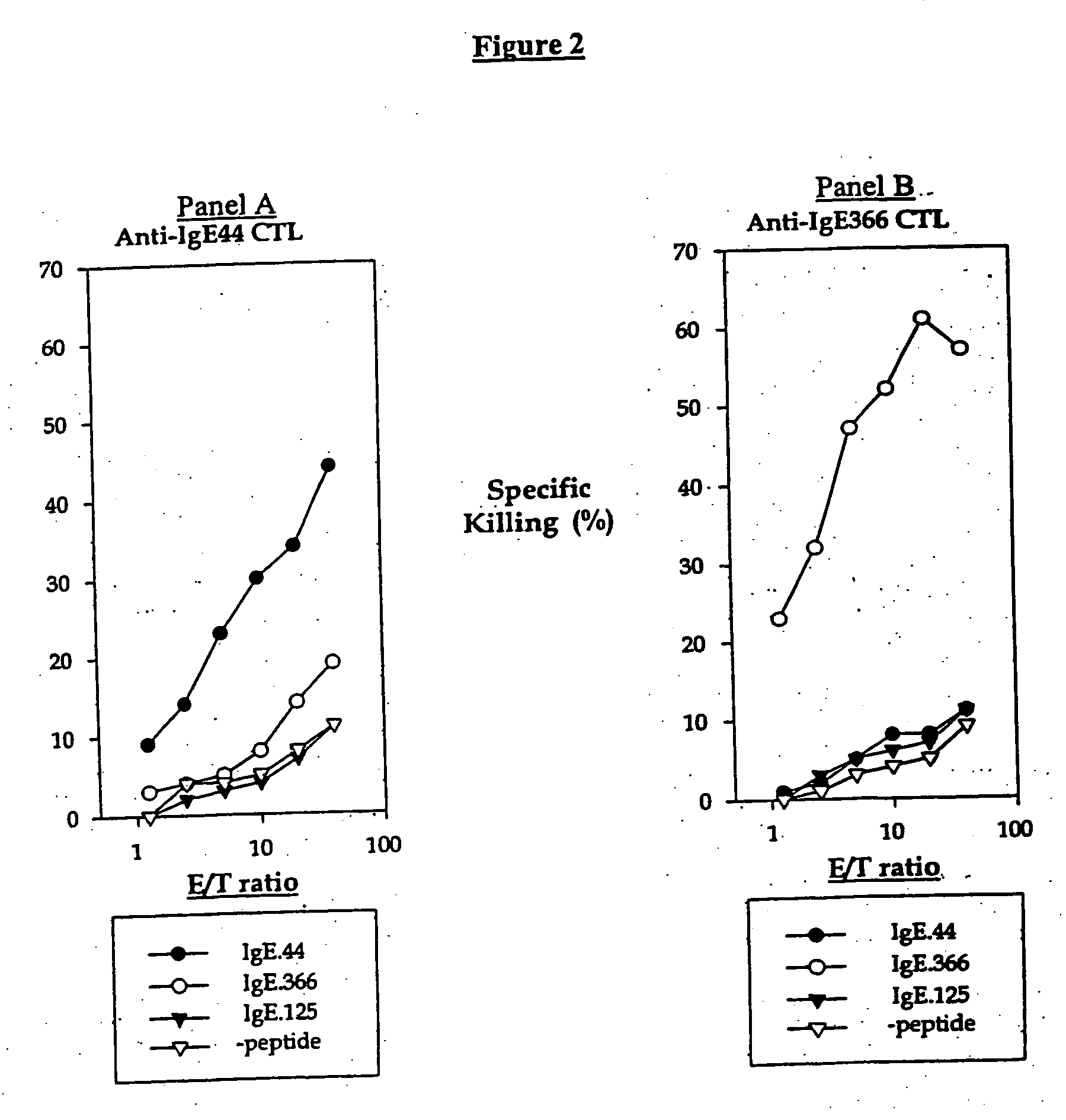
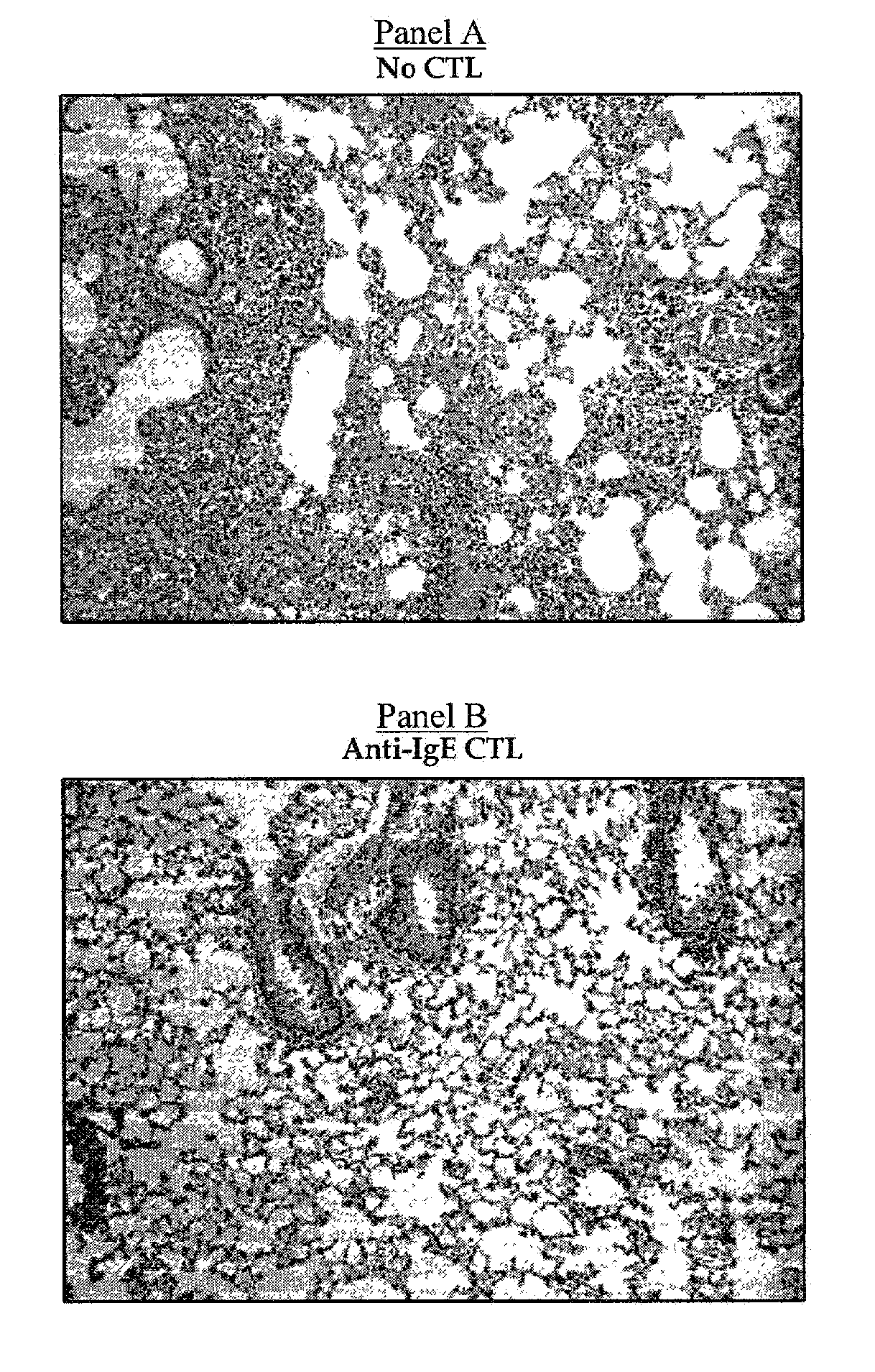
Ex-vivo priming for generating cytotoxic T lymphocytes specific for non-tumor antigens to treat autoimmune and allergic disease
InactiveUS20070098734A1Avoid developmentInhibit progressNervous disorderAntipyreticAbnormal tissue growthDisease cause
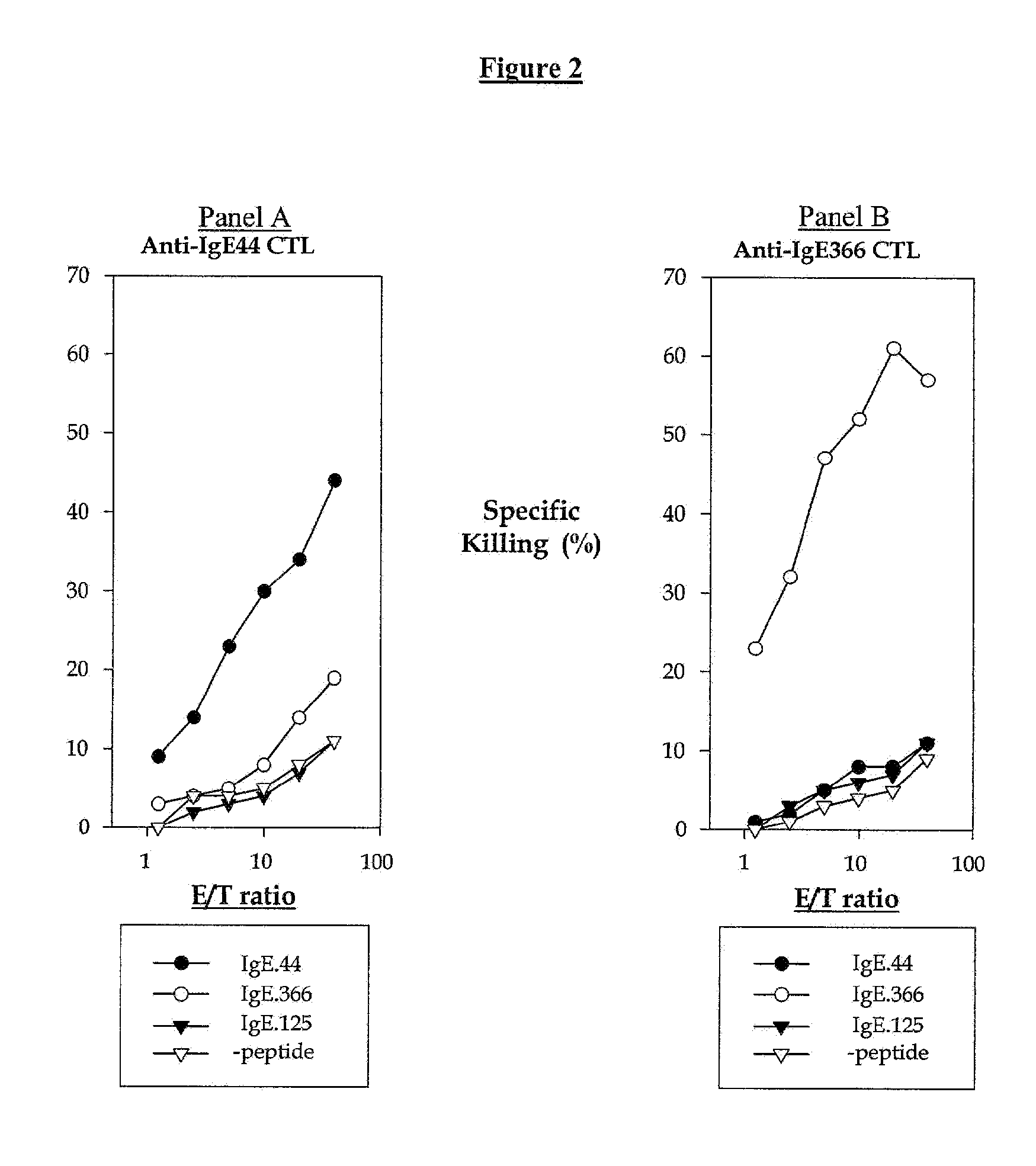
Cytotoxic T lymphocytes (CTLs) specific for antigenic peptides derived from IgE molecule can be generated in vitro by stimulating resting naive CD8 T cells with IgE peptides presented by artificial antigen presenting cells. The IgE specific CTLs lyse the target cells loaded with IgE peptides in vitro and inhibit antigen specific IgE response in vivo. In addition, adoptive transfer of the IgE specific CTL to an asthmatic mouse model can inhibit the development of lung inflammation and airway hypersensitivity. IgE specific CTL provides a treatment for allergic asthma and other IgE-mediated allergic diseases. Antigenic peptides identified from non-tumor self-antigens induce specific cytotoxic T lymphocyte (CTL) in vitro. The CTL induced by peptides identified from CD40L can kill activated CD4 T cells. In vitro generated CTL specific for CD40L inhibit CD4-dependent antibody responses of all isotypes in vivo. In contrast, CTL induced by antigenic peptides derived from IgE specifically inhibit IgE responses, and adoptive transfer of CD40L-specific CTL to NOD mice at early age delay the development of diabetes in NOD mice. In vitro generated CTL specific for non-tumor self-antigens expressed on activated CD4 T cells regulate immune responses in vivo.
Owner:ORTHO MCNEIL PHARM INC
Linear 33 peptide capable of preventing and treating type 1 diabetes and having function of protecting pancreatic beta cells and application of linear 33 peptide
InactiveCN108586619ASuppress immune rebalancingSingle ingredientSenses disorderNervous disorderDiseaseCellular functions
The invention discloses a linear 33 peptide molecule which consists of a B epitope (10 peptide) of human dipeptidyl peptidase 4 and 23 peptide containing a Th2 epitope; serum of an animal immunized by33 peptide contains an antibody which has high price and can be specifically combined with DPP4; the antibody can inhibit the activity of the DPP4 and regulate immune rebalance trending to Th2; the 33 peptide is utilized to carry out subcutaneous immunological control or treat type 1 diabetic NOD mice and treat STZ-induced diabetic mice every 2 weeks, so that blood glucose is significantly controlled or reversed; the blood glucoses of the NOD mice are basically normal, GLP-1 and Th2 cytokines are significantly increased and glucagon and Th1 cytokines are significantly reduced, so that morbidity, death rate, multiple immune balances as well as uric acid, creatinine, blood lipid and oxidative stress indicators are remarkably improved, pancreatic inflammation is relieved, and the ability ofpancreas secreting insulin is significantly strengthened. The 33 peptide as well as vaccines and drugs prepared by using microorganisms, animals, plants and cell expression based on the 33 peptide areof great value in preventing and treating diabetes, especially in type 1 diabetes and complications and other diseases.
Owner:CHINA PHARM UNIV
Compositions for preserving insulin-producing cells and insulin production and treating diabetes
ActiveUS20150191522A1Reduce accumulationEnhancing activation-induced cell deathPolypeptide with localisation/targeting motifPeptide/protein ingredientsAutoimmune diabetesIn vivo
Nuclear Transport Modifiers such as cSN50 and cSN50.1, afford in vivo islet protection following a 2-day course of intense treatment in autoimmune diabetes-prone, non-obese diabetic (NOD) mice, a widely used model of Type 1 diabetes (T1D), which resulted in a diabetes-free state for one year without apparent toxicity and the need to use insulin. cSN50 precipitously reduces the accumulation of islet-destructive autoreactive lymphocytes while enhancing activation-induced cell death of T and B lymphocytes derived from NOD mice. cSN50 attenuated pro-inflammatory cytokine and chemokine production in immune cells in this model of human T1D. cSN50 also provides cytoprotection of beta cells, therefore preserving residual insulin-producing capacity. Because intracellular delivery of a Nuclear Transport Modifier peptide such as cSN50 and cSN50.1 can result in lowering of fasting blood glucose levels and may ameliorate (e.g., reduce, eliminate) insulin resistance, the compositions, methods and cells described herein can also be used for treating Type 2 diabetes (T2D).
Owner:VANDERBILT UNIV
Non-obese diabetes mice and its applications
ActiveUS10634666B2Stable expressionConveniently and accurately tracingImmunoglobulinsBiological testingDiabetes mellitusNon obese
The invention relates to a novel non-obese diabetic (NOD) mouse and its application. Particularly, the invention relates to a NOD mouse specifically expressing anti-polyethylene glycol membrane antibody reporter (anti-PEG reporter) in the NOD mouse.
Owner:TAIPEI MEDICAL UNIV
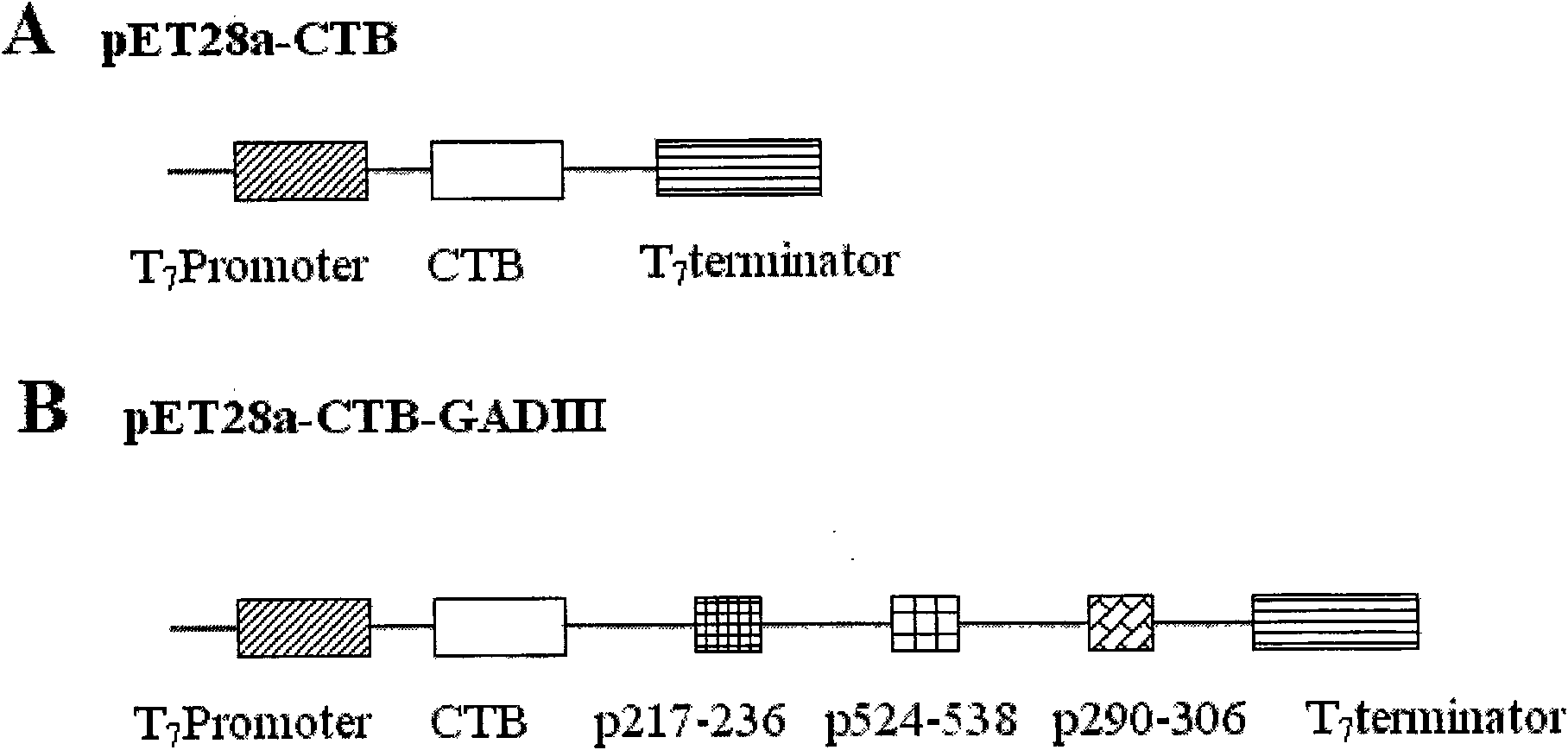
Adjuvant-free immunity regulating agent for preventing and treating type 1 diabetes
InactiveCN101574517AReduce morbidityLowered humoral immunityMetabolism disorderCarrier-bound antigen/hapten ingredientsAntigen epitopeAdjuvant
The invention provides an adjuvant-free immunity regulating agent for preventing and treating type 1 diabetes. Polypeptide with single antigen is hard to re-establish immunological tolerance and block self-immune attack after an organism has received the self-immune attack. Three antigen epitope polypeptide genes (stemmed from a human glutamate decarboxylase 65 molecule) are orderly connected in series and connected to a lower reach of a cholera toxin B subunit gene so as to realize the fusional expression of the serially connected antigen epitope polypeptides and CTB. Without adding any adjuvant, the obtained fusion protein can excite the organism to generate the immunological tolerance aiming at body fluid of the serially connected antigen polypeptides by a way of mucous membrane immunity and obviously lower the occurrence of the NOD mouse diabetes, so that the immunity regulating agent can be used for preventing and treating the type 1 diabetes.
Owner:CHINA PHARM UNIV
Design and application of novel diabetes immunomodulatory peptide-vp peptide
ActiveCN106518987BChange spaceGood hypoglycemic effectPeptide/protein ingredientsMetabolism disorderSide effectSerum ige
A novel diabetes-related immunoloregulation vaccine peptide VP is designed which can effectively prevent T1DM attack and is free of an atherosclerotic side effect. The VP peptide does not cause lipid disorders in serum of mice, sharp hs-CRP level increases or atherosclerosis side effects. The VP peptide can control blood glucose of NOD mice, maintain normal body weights of mice and reduce the incidence of T1DM. In addition, the VP peptide can maintain normal secretion functions of islet beta cells and can allow the cells to exert normal blood sugar adjusting functions.
Owner:CHINA PHARM UNIV
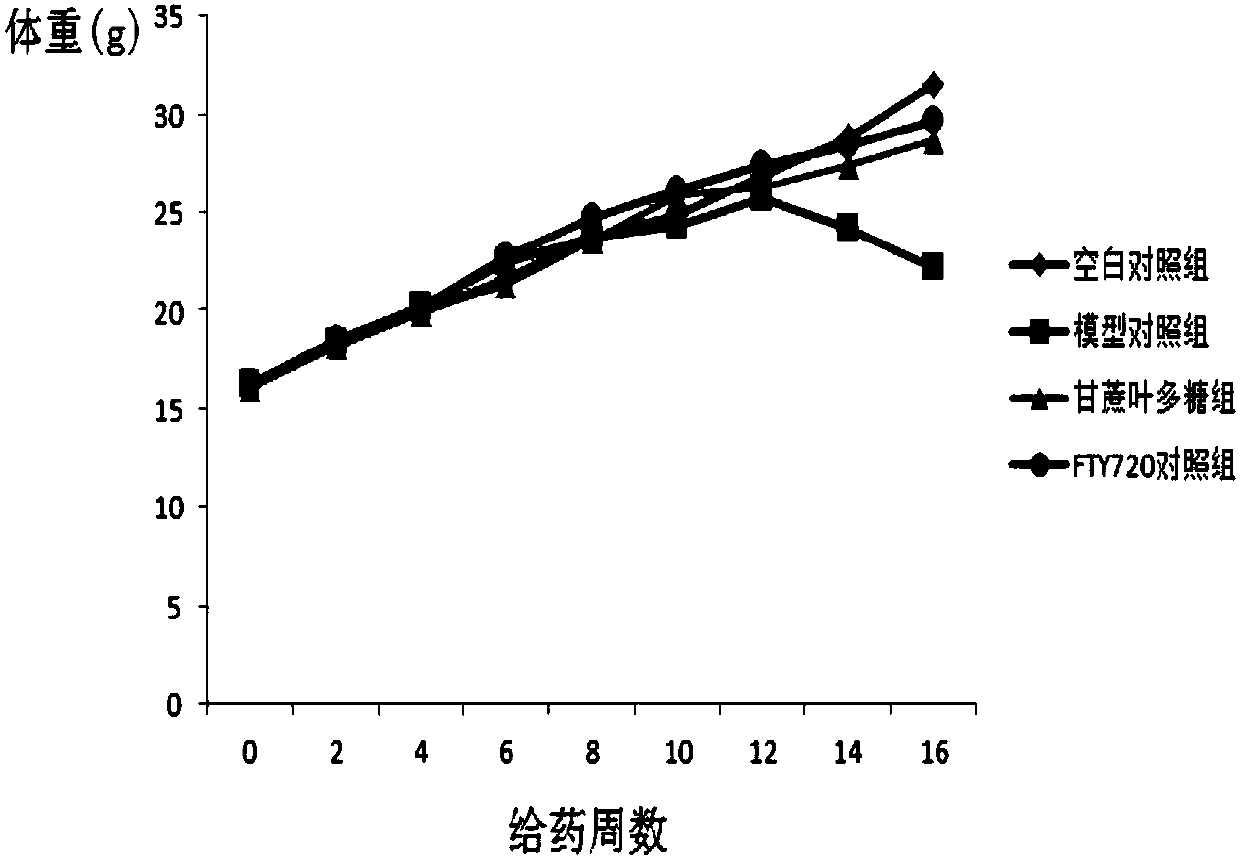
Preparation of sugarcane leaf polysaccharide and its application to treat and prevent diabetes
InactiveCN107778374AImprove inflammatory responseImprove antioxidant capacityOrganic active ingredientsMetabolism disorderAntioxidant capacityMedicine
The invention discloses a preparation method of sugarcane leaf polysaccharide and its application to treat and prevent diabetes. An inventor extracts and acquires sugarcane leaf polysaccharide from sugarcane leaves; the inventor carries out deep study by adopting non-obese diabetic mice, and finds that the sugarcane leaf polysaccharide has preventive effect to the mice diabetes of the non-obese diabetic mice. The experiment result shows that there are 8 mice with diabetes from 12 NOD mice of a model group, wherein the morbidity is 66.7%; there are 3 mice with diabetes from 12 NOD mice of a sugarcane leaf polysaccharide group, the morbidity is 25%; the morbidity is obviously reduced (P is less than 0.05); the mechanism may be related to the sugarcane leaf polysaccharide ability of improvinginflammatory reaction in the NOD mice body and enhancing the oxidization resistance in the NOD mice body. Hereby, the sugarcane leaf polysaccharide has a certain application prospect in treating andpreventing diabetes, in particular to I-type diabetes.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Application of recombinant lactic acid bacteria and snase in the preparation of drugs for preventing or treating type 1 diabetes
ActiveCN105770868BHas hypoglycemic effectAvoid degradationPeptide/protein ingredientsHydrolasesProtein targetPharmacodynamic Study
The invention relates to the application of recombinant lactic acid bacteria and SNase in the preparation of drugs for preventing or treating type 1 diabetes. The purpose of the present invention is to provide the new application of the vaccine with recombinant lactic acid bacteria L.lactisNZ9000 pCYT:SNase as carrier in anti-type 1 diabetes. The recombinant bacteria can express the target protein Staphylococcal nuclease, SNase after being induced by lactose. L.lactisNZ9000 pCYT:SNase live bacterial vaccine was immunized to 4-week-old female NOD / LtJ mice by oral administration, and a series of pharmacodynamic indicators were evaluated. It was found that: compared with the control group, L.lactisNZ9000 pCYT:SNase can significantly reduce the incidence of diabetes in NOD mice, better control the blood sugar and body weight of NOD mice, and significantly improve the survival rate of NOD mice. It can be seen that the recombinant Lactococcus lactis L. lactisNZ9000 pCYT:SNase can significantly inhibit the development of diabetes in NOD mice, and can be used for the development of type 1 diabetes drugs.
Owner:CHINA PHARM UNIV
Expression method of recombinant human insulin, special expression vector, engineering bacteria and application
The invention provides an expression method of recombinant human insulin, a special expression vector, engineering bacteria and application. This expression method can induce the expression of recombinant human insulin gene in food-grade lactic acid bacteria and display recombinant human insulin on the surface of lactic acid bacteria. The engineering bacteria, expression vector and inducer used in this expression method all meet the requirements of food grade, avoiding the The presence of resistance genes on vectors and the potential harm caused by non-food-grade inducers, etc. The lactic acid bacteria engineering bacteria containing cell wall display insulin obtained by the present invention can stimulate NOD mice to produce recombinant human insulin-specific antibodies, significantly increase the level of cytokine IL-4 related to immune tolerance, and induce immune tolerance. The induced lactic acid bacteria engineered bacteria can be used as an oral vaccine for type 1 diabetes after being made into bacterial preparations, without the need for tedious post-processing such as purification, and has broad application prospects.
Owner:WUHAN ZHENFU PHARMA CO LTD
Ex-vivo priming for generating cytotoxic t lymphocytes specific for non-tumor antigens to treat autoimmune and allergic disease
InactiveUS20090202506A1Avoid developmentInhibit progressBiocideNervous disorderDiseaseAutoimmune responses
Owner:ORTHO MCNEIL PHARM INC
Recombinant protein and recombinant gene capable of preventing and curing I type diabetes mellitus through immunity
InactiveCN100352502CReduce immune damageMetabolism disorderAntibody ingredientsAntigen epitopeAntigen
The present invention provides one kind of restructuring protein and its restructuring gene for preventing and treating type-1 diabetes. Connecting heat shock protein HSP60 originated Mycobacterium bovine to a piece of antigen epitope P277 from human HSP60; restructuring protein produced can be used for immunity directly with no adjuvant. The restructuring protein can stimulate body to produce antigen for P277 both by ways of injection and mucous membrane immunity, decreases marked NOD mouse diabetes occur, can contribute to preventing and caring type-1 diabetes. Meantime, the restructuring gene produced in this invention can also be used to transgene animals or plants and restructuring microorganism as the factory for producing restructuring protein or preparing oral vaccine.
Owner:CHINA PHARM UNIV
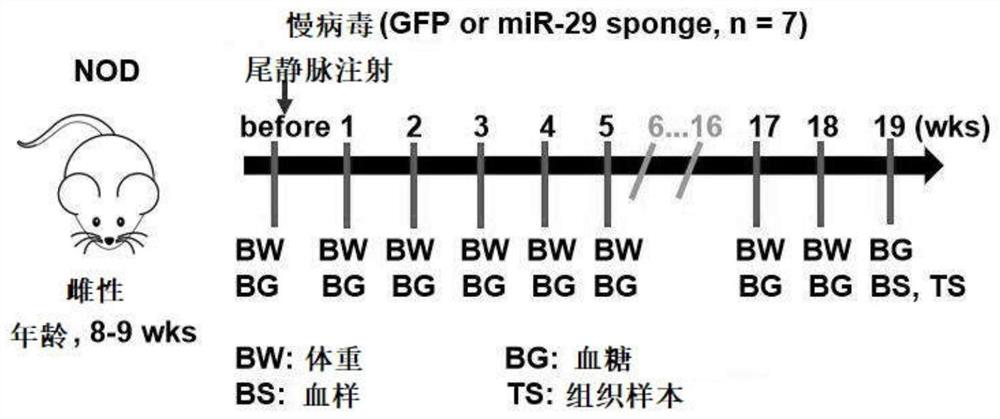
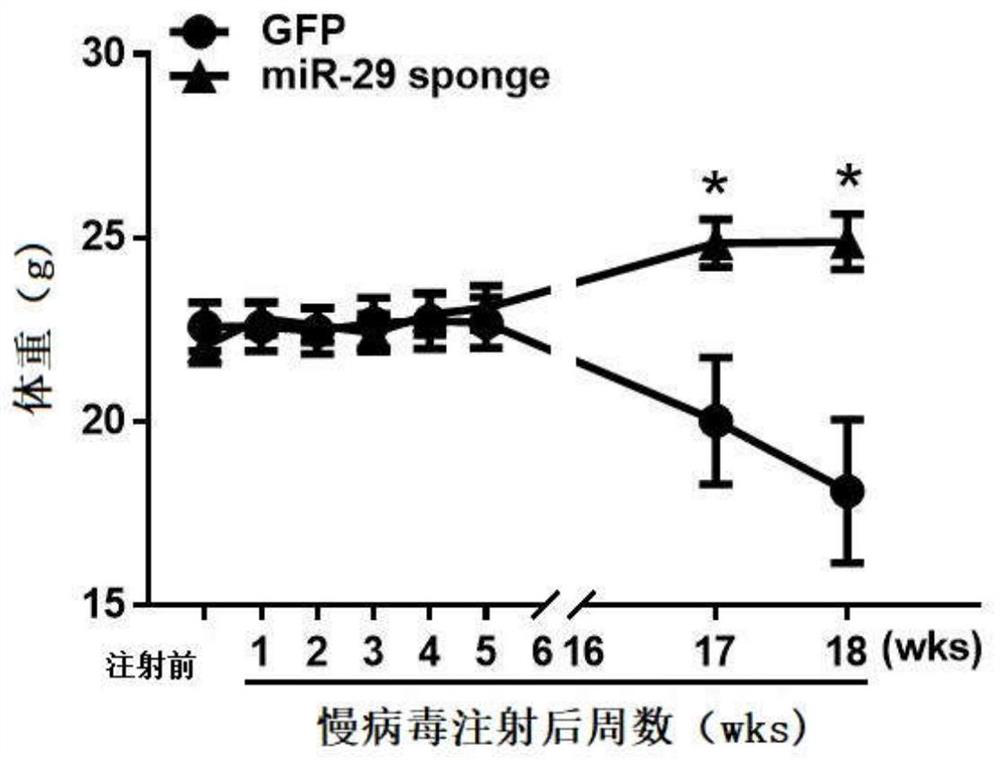
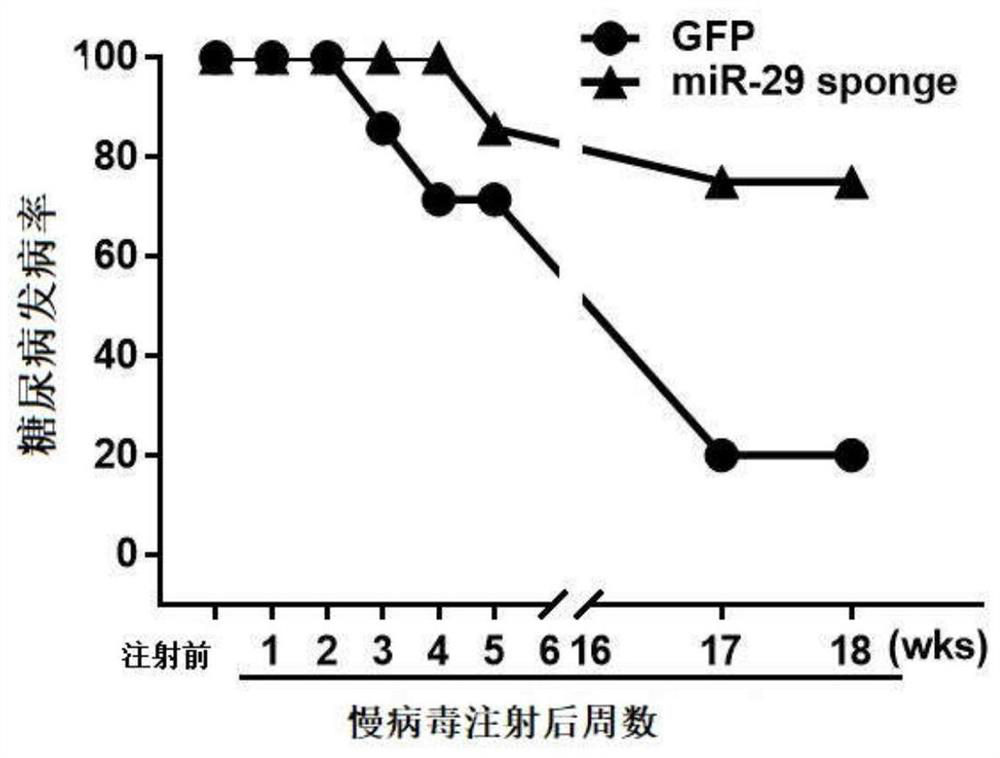
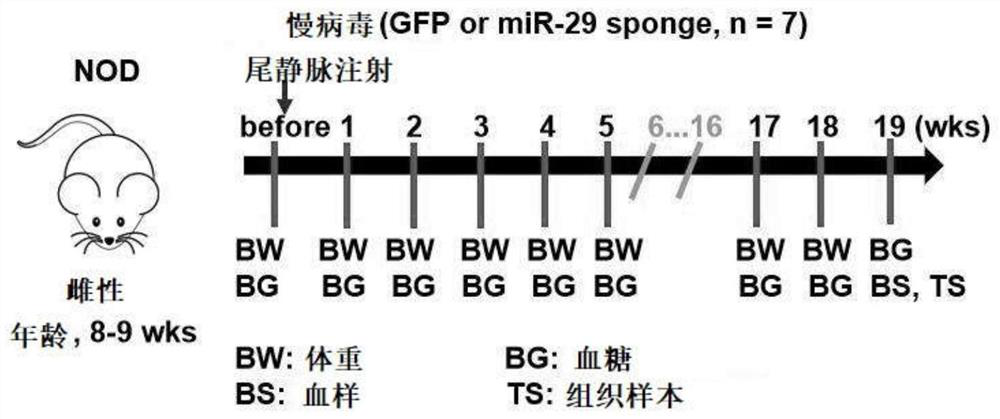
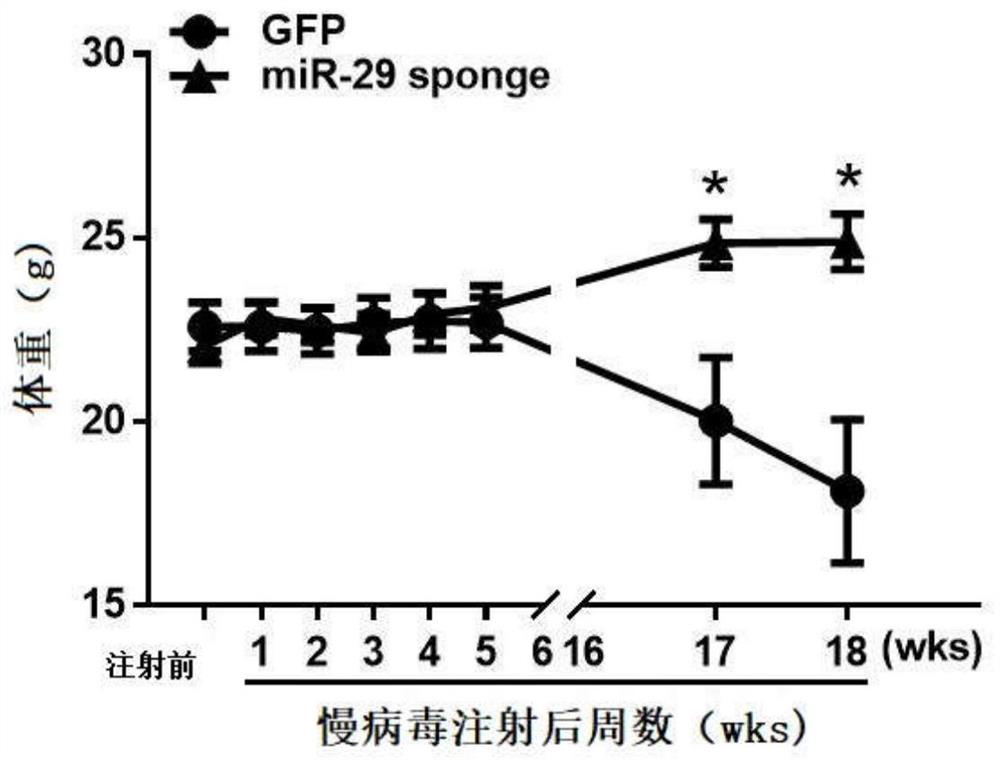
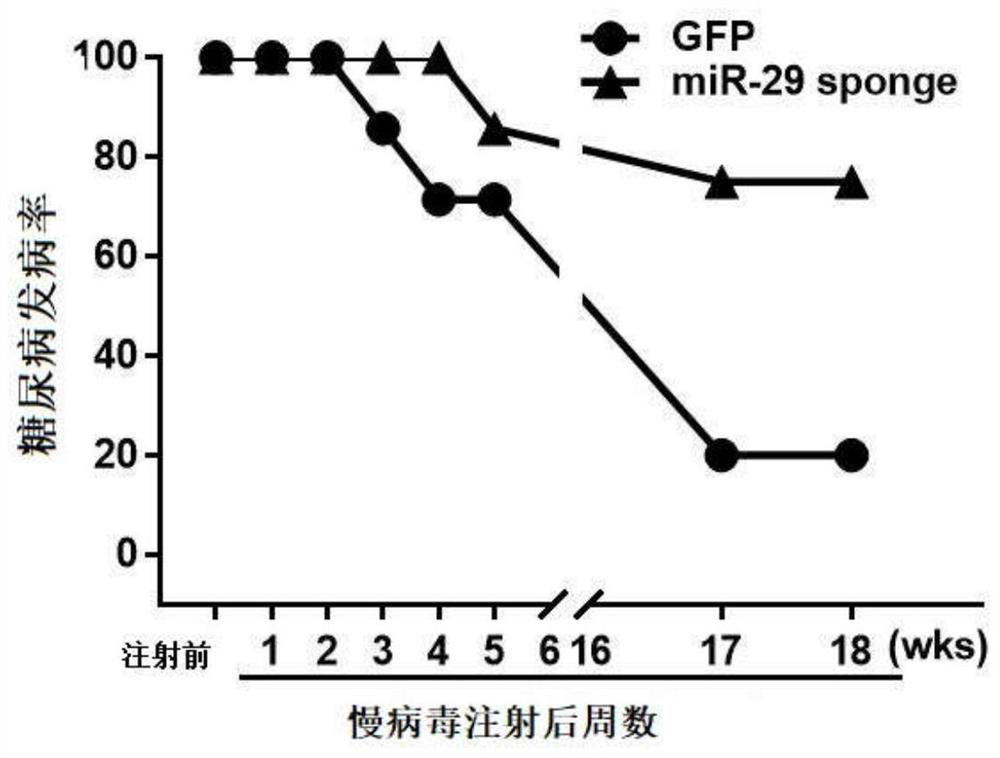
MiR-29 sponge, nucleic acid construct containing same and applications of miR-29 sponge
ActiveCN112226436AInhibition of secretionImprove survival rateOrganic active ingredientsSpecial deliveryPhysiologyNod mouse
The invention discloses a miR-29 sponge, and further provides a nucleic acid construct containing the same. The invention finds that the miR-29 sponge can adsorb miR-29a-3p and miR-29b-3p in the body,close the functions of the miR-29a-3p and the miR-29b-3p and inhibit secretion of pro-inflammatory cytokines, so that insulitis of NOD mice is relieved, the serum insulin level is increased, the blood glucose tends to be normal, and finally, the onset of Type 1 diabetes mellitus (T1DM) of NOD (non-obese diabetic) mice is remarkably reduced, and the survival rate of the NOD mice is increased.
Owner:NANJING MEDICAL UNIV
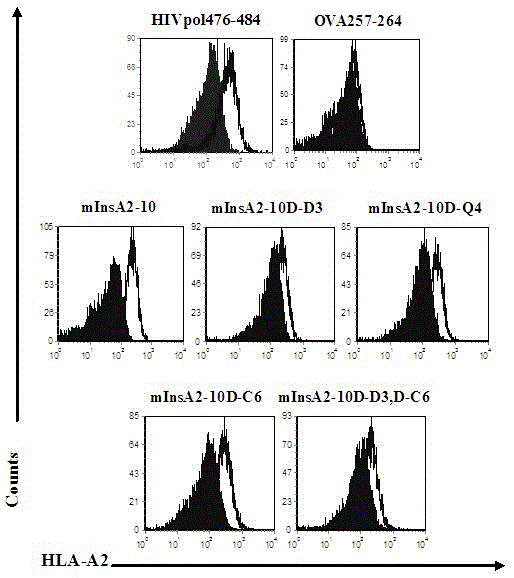
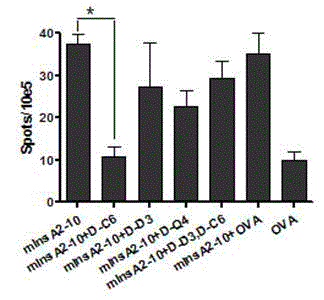
Insulin chain A human leukocyte antigen (HLA)-A*0201 restrictive cytotoxic T lymphocyte (CTL) epitope altered peptide ligand and application thereof
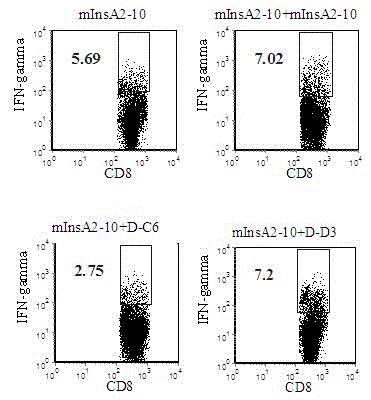
The invention discloses an insulin chain A human leukocyte antigen (HLA)-A*0201 restrictive cytotoxic T lymphocyte (CTL) epitope altered peptide ligand (APL) which is obtained by replacing sixth amino acid L-Cys in insulin chain A HLA-A*0201 restrictive natural CTL epitope mInsA2-10 of humanized non-obese diabetic (NOD) mice with D-Cys. The amino acid sequence of the APL is shown as SECIDNo.1L; the natural CTL epitope has high cross reactivity with human epitope, and the APL has an obvious characteristic of inhibiting natural epitope specificity CTL response in the extracorporal cellar immunefunction experiment of the humanized NOD mice and the peptide has the characteristics that the peptide is easy to prepare and purify in a large scale and can be used safely, so that the APL can be independently applied to the development of I-type diabetes mellitus therapeutic antagonist peptide vaccine or is combined with other immunodominance autoantigen epitopes or APL to be applied to the development of I-type diabetes mellitus therapeutic antagonist peptide vaccine, and has potential and good development and application prospect in the field of human I-type diabetes mellitus treatment.
Owner:ARMY MEDICAL UNIV
mir-29 sponge, nucleic acid construct comprising mir-29 sponge and application thereof
ActiveCN112226436BInhibition of secretionImprove survival rateOrganic active ingredientsSpecial deliveryNod mousePancreatic hormone
The invention discloses a miR-29 sponge, and further provides a nucleic acid construct comprising the miR-29 sponge. The present invention finds that the miR-29 sponge can absorb miR-29a-3p and miR-29b-3p in the body and close its function, inhibit the secretion of pro-inflammatory cytokines, thereby alleviating the occurrence of insulitis in NOD mice, increasing serum insulin levels, maintaining The blood sugar tends to normal level, finally significantly reduces the incidence of type 1 diabetes in NOD mice, and improves the survival rate of NOD mice.
Owner:NANJING MEDICAL UNIV
Compositions and methods for preserving insulin-producing cells and insulin production and treating diabetes
Nuclear Transport Modifiers such as cSN50 and cSN50.1, afford in vivo islet protection following a 2-day course of intense treatment in autoimmune diabetes-prone, non-obese diabetic (NOD) mice, a widely used model of Type 1 diabetes (T1D), which resulted in a diabetes-free state for one year without apparent toxicity and the need to use insulin. cSN50 precipitously reduces the accumulation of islet-destructive autoreactive lymphocytes while enhancing activation-induced cell death of T and B lymphocytes derived from NOD mice. cSN50 attenuated pro-inflammatory cytokine and chemokine production in immune cells in this model of human T1D. cSN50 also provides cytoprotection of beta cells, therefore preserving residual insulin-producing capacity. Because intracellular delivery of a Nuclear Transport Modifier peptide such as cSN50 and cSN50.1 can result in lowering of blood glucose levels and may reducing insulin resistance, the compositions, methods and cells described herein can also be used for treating Type 2 diabetes (T2D).
Owner:VANDERBILT UNIV
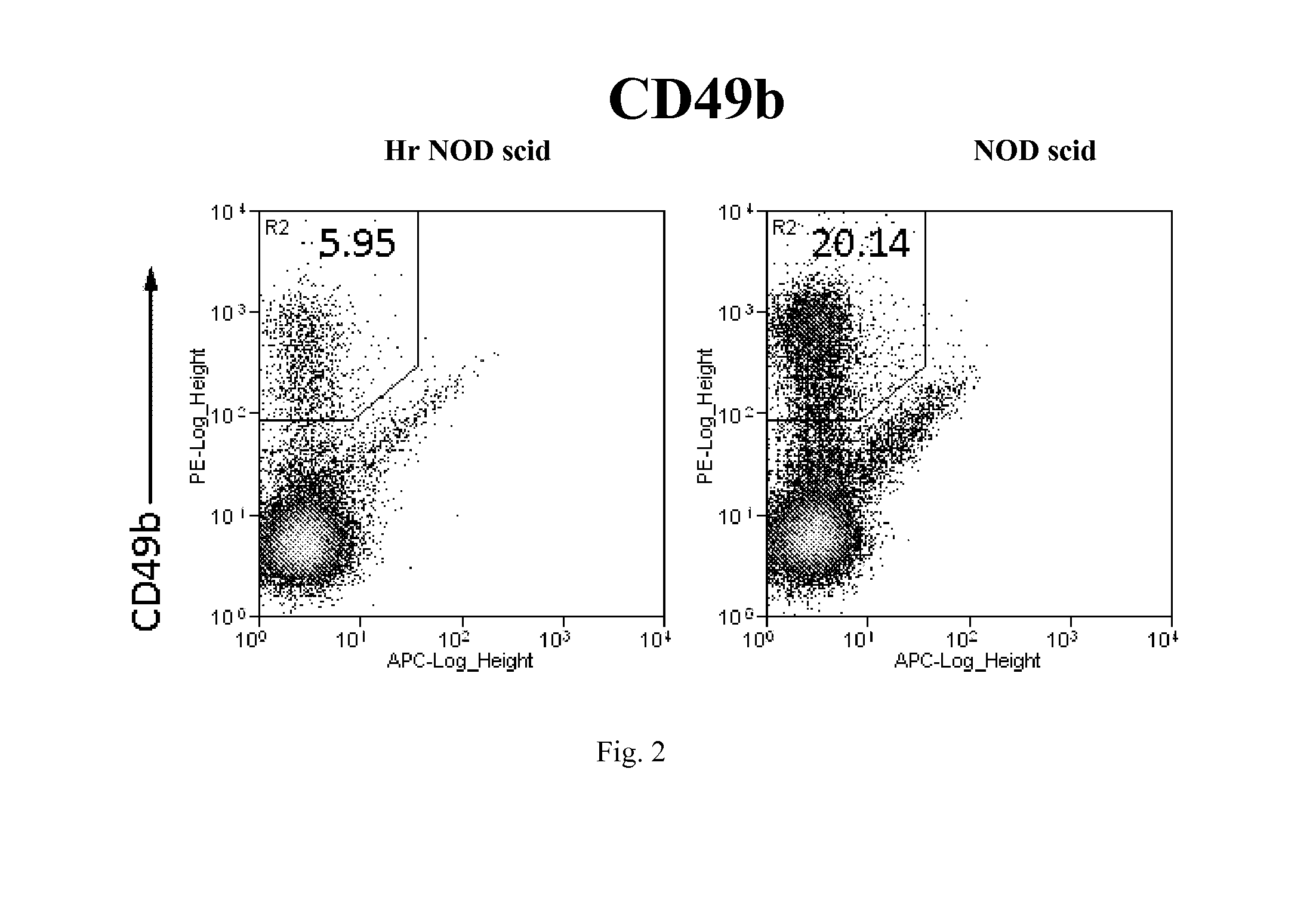
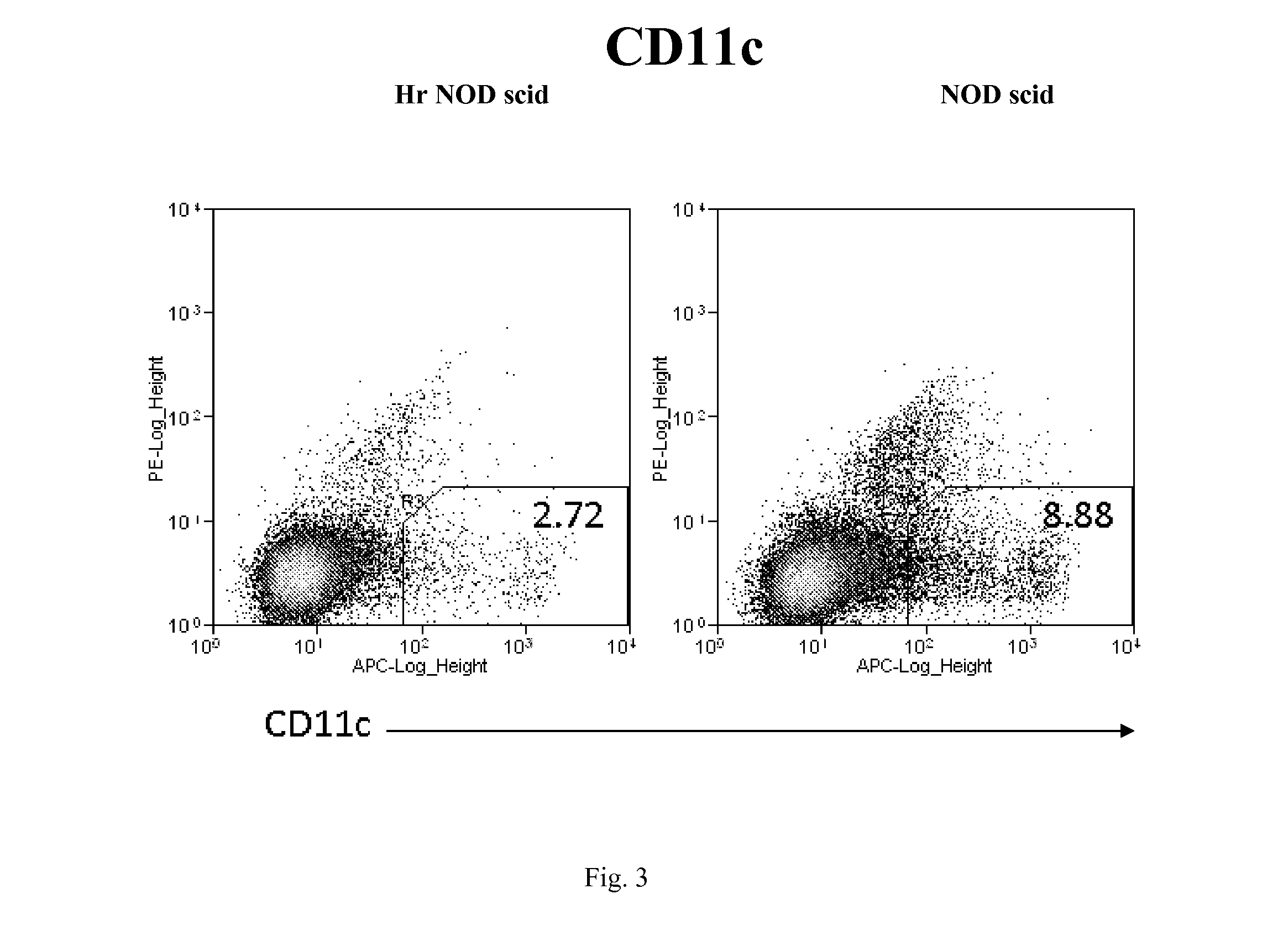
Hairless nod scid mouse
Hairless, immunodeficient mice on a non-obese diabetic (NOD) background and methods for their production are disclosed herein. The mice are hairless and have multiple immunodeficiencies, including B-cell and T-cell deficiencies, as well as impaired macrophage and complement function. The mice also have a further deficit in natural killer and dendritic cells of the immune system. The mice are useful for biomedical research, for example, in studies involving xenograft transplantation, spontaneous tumors, cancer cell tumorigenesis, tumor angiogenesis, tumor metastatic potential, tumor suppression therapy, carcinogenesis regulation, and tumor imaging.
Owner:ENVIGO RMS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com