Adjuvant-free immunity regulating agent for preventing and treating type 1 diabetes
An immunomodulator, type 1 diabetes technology, applied in DNA recombination technology, protein separation and purification technology and medical related fields, can solve the problems of loss of drug activity, limited application prospects, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
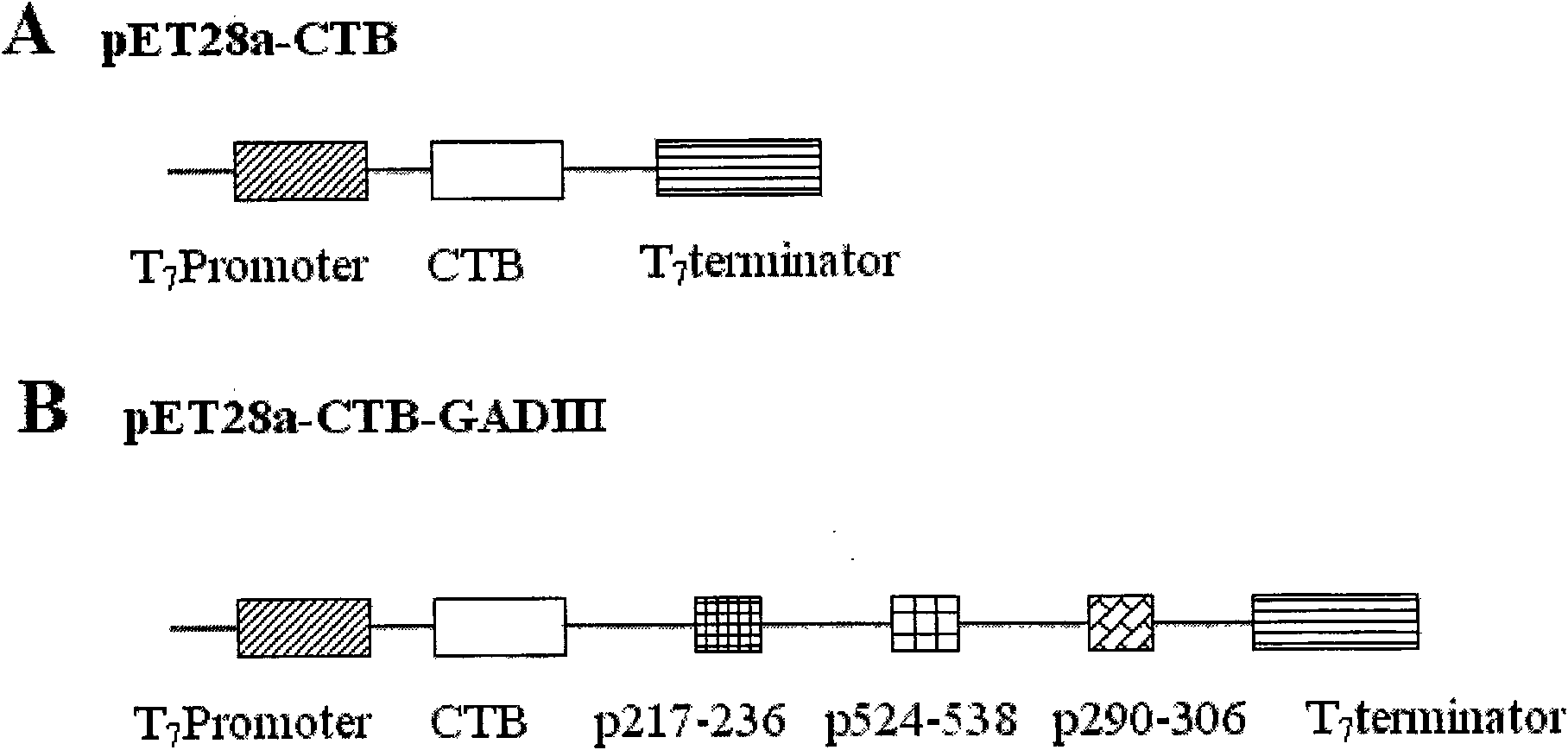
[0046] Embodiment 1: Design, synthesis and cloning of CTB-GADIII polypeptide gene
[0047] According to the amino acid sequence of CTB gene and GAD65 antigen peptide gene, Escherichia coli preferred codons were selected, and six oligonucleotide fragments were designed by computer, and GADIII polypeptide gene was first synthesized by PCR method, and the gene was cloned into the downstream of pET28a-CTB gene , transformed into Escherichia coli to obtain a recombinant plasmid named pET28a-CTB-GADIII. The specific method is as follows:
[0048] Six oligonucleotides:
[0049] P1: 5′-AAA GCTAG CGGTGGTGAATACGTTACTCTGAAAAAAATGCGTGAAATCATCGGTT-3′
[0050] P2: 5′-AAAAAGCTTAGTCACCTGAACCACCCGGCCAACCGATGATTTCACGCATT-3′
[0051] P3: 5′-GATAACCGGAGCAACTTTAGACAGACGTGAGTCACCTGAACCACCCGGCCAA-3′
[0052] P4: 5′-AAAAAGCTTACATCATACGAGCTTTGATAACCGGAGCAACTTTAGAC-3′
[0053] P5: 5′-AACGGAGTCAGTACCGATACCCAGAGCAGCATCATACGAGCTTTGATAA-3′
[0054] P6: 5′-AAA AAGCTT ATTCGTCGCATTTGATTAAGATAACGGAGT...
Embodiment 2
[0058] Embodiment 2: Expression of CTB-GADIII gene in Escherichia coli
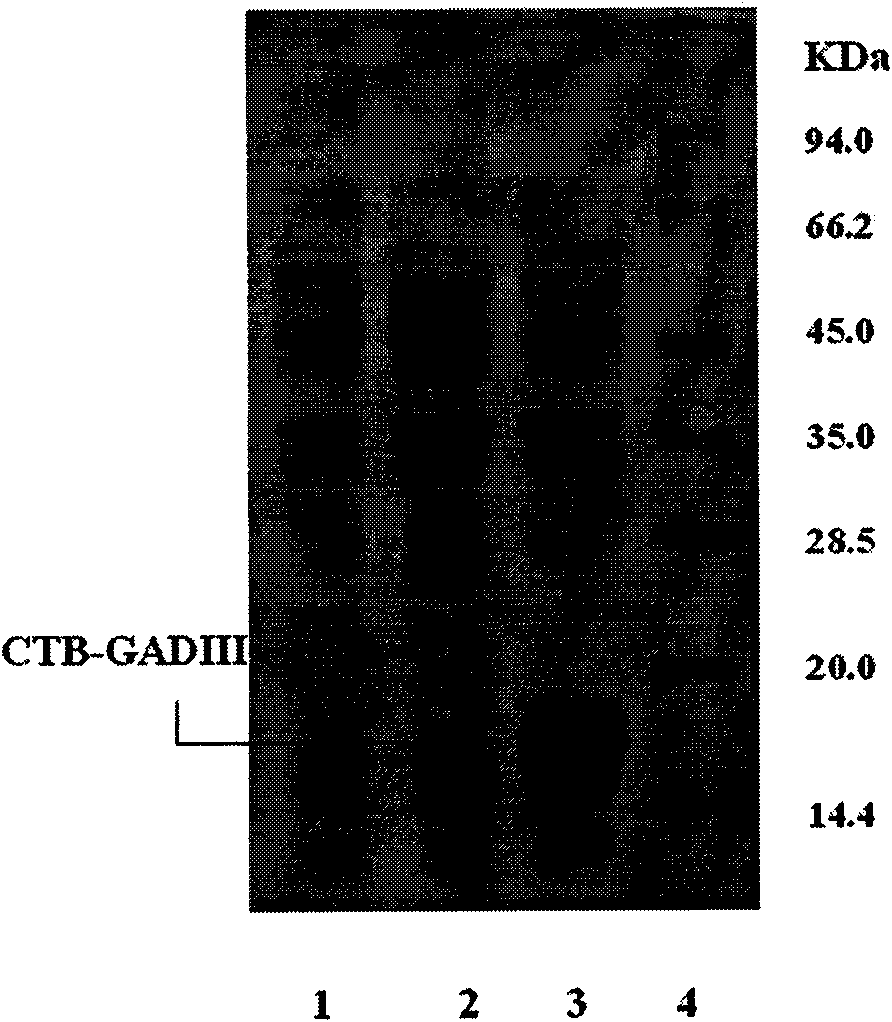
[0059] The recombinant plasmid pET28a-CTB-GADIII was transformed into Escherichia coli BL21. Pick a single colony from a plate with different transformants and inoculate it into LB liquid medium containing 50 μg / ml kanamycin. After culturing at 37°C for 4 hours, add α-lactose at a final concentration of 5 mmol / L to induce E. coli to express T 7 RNA polymerase, continue culturing to express the fusion protein CTB-GADIII. After induction, a small amount of bacterial liquid was taken every 1 hour, and the bacterial cells were recovered by centrifugation. SDS-PAGE electrophoresis and thin-layer scanning showed that the fusion expression of the CTB-GADIII polypeptide gene had been realized. The fusion protein reaches a stable maximum expression level after 6 hours of induction, and the fusion protein accounts for more than 50% of the total bacterial protein. The results are shown in image 3 .
Embodiment 3
[0060] Embodiment 3: Separation and purification of recombinant protein CTB-GADIII
[0061] Inclusion bodies were washed 3 times with PBS buffer containing 1% Triton. The initially purified fusion protein CTB-GADIII was dissolved in 0.1 mol / L Tris buffer solution (pH 8.9) containing 8 mol / L urea. The denatured protein was isolated and purified using CM52 cation exchange resin. It was eluted with a Tris / NaCl gradient containing 8 mol / L urea, collected in sections for SDS-PAGE electrophoresis detection, and CTB-GADIII was in the elution peak at 120-150 mmol NaCl. The obtained purified protein was dissolved in the renaturation buffer containing 0.1mol / L Tris-HCl, pH 8.9, 0.4mol / L NaCl and 2mmol / l EDTA, and incubated overnight at 37°C for renaturation. The refolded protein was further separated from monomers and pentamers by molecular sieves. Check its purity by SDS-PAGE electrophoresis, see Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com