Recombinant protein and recombinant gene capable of preventing and curing I type diabetes mellitus through immunity
A recombinant protein and diabetes technology, applied in the field of recombinant protein, can solve the problems that it cannot be used in the human body, and the peptide vaccine cannot strengthen the immunogenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
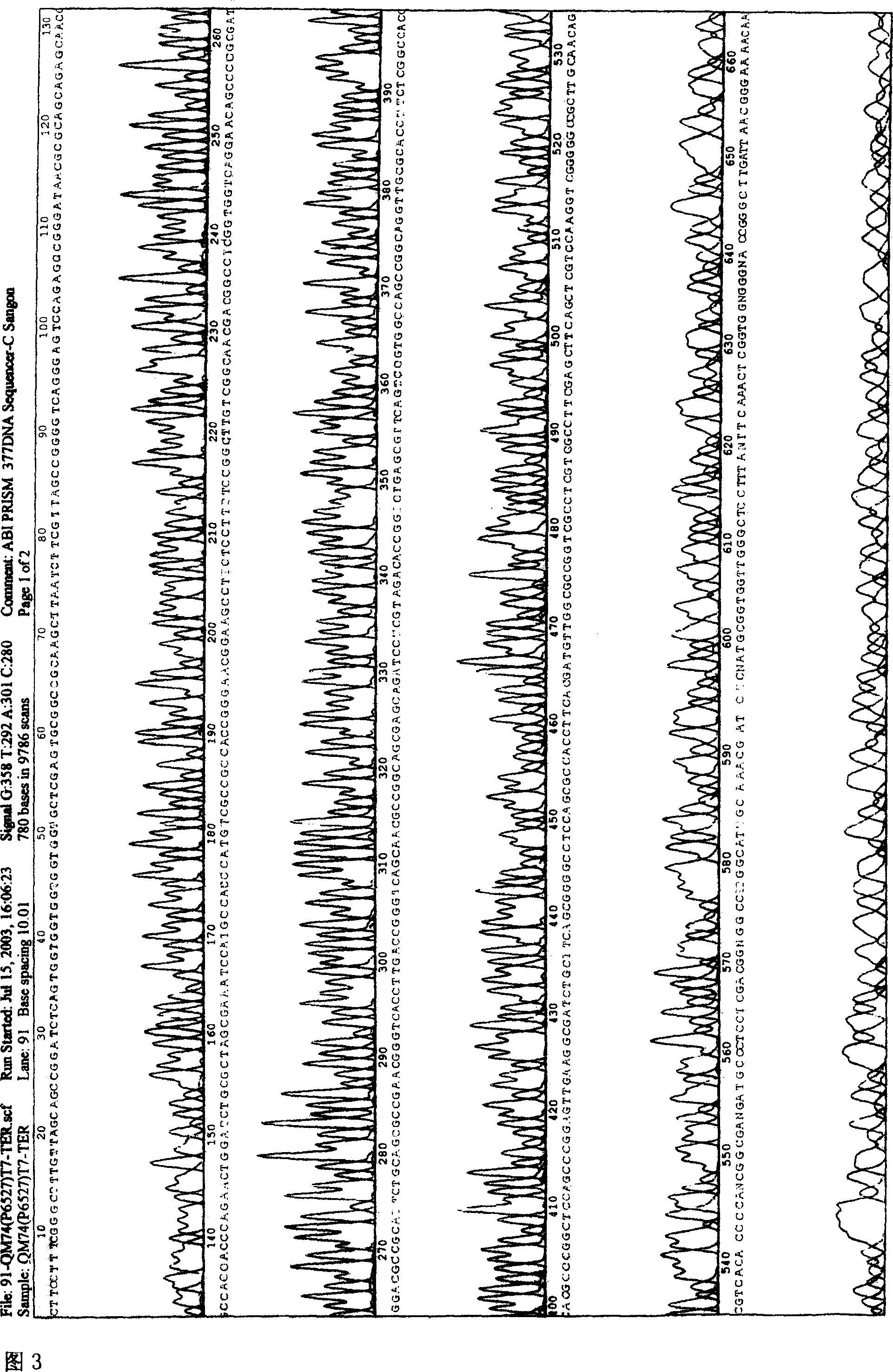
[0056] Example 1 Design, synthesis and cloning of HSP65-P277 polypeptide gene
[0057] According to the amino acid sequences of the MT-HSP65 gene and the P277 polypeptide gene, four oligonucleotide fragments were designed with the aid of a computer by selecting codons favored by Escherichia coli. First, the P277 polypeptide gene was synthesized by PCR method, the gene was cloned into the C-terminus of the L-ansB-C gene of pED, and transformed into Escherichia coli to obtain a recombinant plasmid named pED-P277. Using the pET28a-HSP65 plasmid as a template, the MT-HSP65 gene was obtained by PCR, and the gene was replaced by the L-ansB-C gene in pED-P277, and transformed into Escherichia coli to obtain a recombinant plasmid named PED-HSP65-P277. The specific method is as follows:
[0058] 4 oligonucleotides:
[0059] P1: 5'GCTGGATCCAGTTCTGGGTGGTGGCGTTGCTCTGCTGCGCGTTATCCCGGCTCTGG3'
[0060]P2: 5'GTCAAGCTTAATCTTCGTTAGCCGGGGTCAGGGAGTCCAGAGCCGGGATAACGCGC 3'
[0061] P3: 5’TTG AC...
Embodiment 2
[0065] Example 2 Expression of HSP65-P277 gene in Escherichia coli
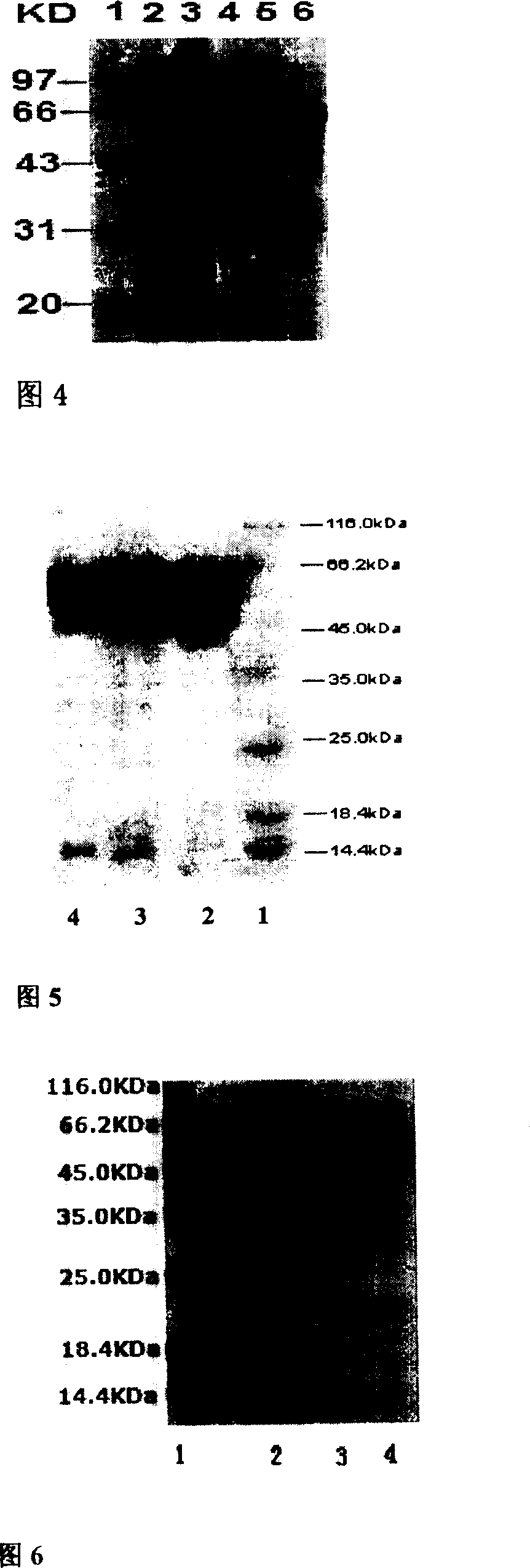
[0066] The recombinant plasmid pED-HSP65-P277 was transformed into Escherichia coli BL21. Pick single bacterium colony and inoculate containing 50ug / ml kanamycin LB liquid culture medium from growing different transformant plates, cultivate overnight at 37 DEG C of constant temperature shaking, transfer and inoculate into fresh corn steep liquor liquid culture medium by 1% ratio ( 50ug / ml kanamycin), after culturing at 37°C for 4 hours, adding α-lactose at a final concentration of 0.5mmol / L to induce E. coli to express T 7 RNA polymerase, continue to culture to express the fusion protein HSP65-P277. After induction, a small amount of bacterial liquid was taken every 1 hour, and the bacterial cells were recovered by centrifugation. SDS-PAGE electrophoresis and thin-layer scanning showed that the fusion expression of the HSP65-P277 polypeptide gene had been realized. The fusion protein reached a stable maximu...
Embodiment 3
[0067] Example 3 Separation and Purification of Recombinant Protein HSP65-P277
[0068] The engineered bacteria after induced expression were collected by centrifugation, suspended in the cell lysate (pH 8.0, 50mM phosphate buffer, 0.02% lysozyme), stirred at 37°C for 30 minutes, and DNase was added to digest the DNA Until the solution is not viscous, the supernatant is recovered by centrifugation, and ammonium sulfate is used for fractional precipitation. The target protein is mainly in the 35%-45% ammonium sulfate precipitation, as shown in Figure 5. The precipitated fusion protein was redissolved in pH 7.4 phosphate buffer, dialyzed for desalting, and after centrifugation, the supernatant was taken for anion exchange column chromatography, DEAE cellulose DE-52 column (2×60cm), using PBS / NaCl gradient Elution, fractional collection for SDS-PAGE electrophoresis detection, HSP65-P277 in the elution peak at 120-150mmol NaCl, check the purity of the prepared samples by SDS-PAGE ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com