Application of recombinant lactic acid bacteria and snase in the preparation of drugs for preventing or treating type 1 diabetes
A type 1 diabetes and Lactococcus lactis technology, applied in the direction of drug combination, microbial-based methods, microbial measurement/testing, etc., can solve the problem of no report of hypoglycemia, avoid cumbersome and complicated purification steps, and save production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
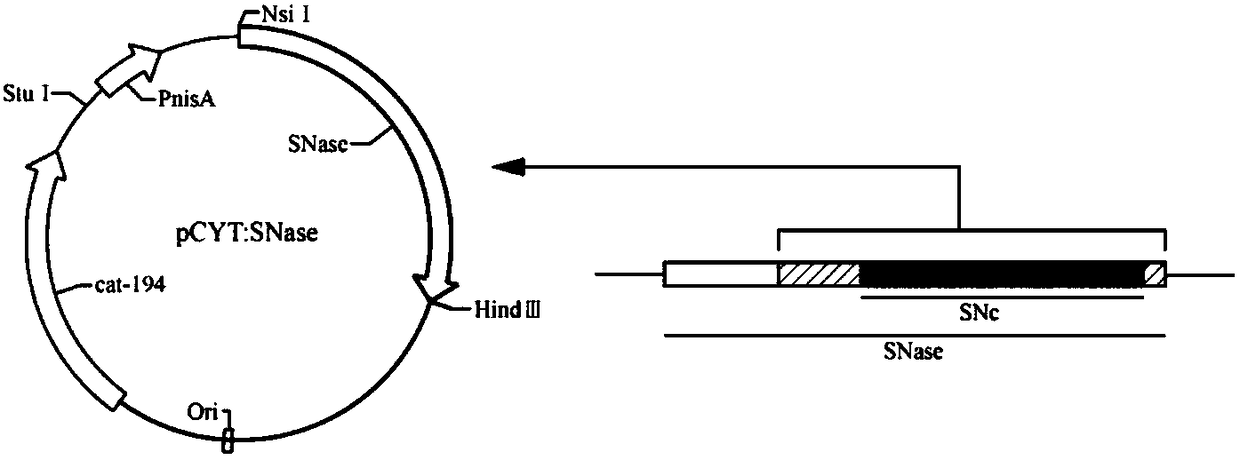
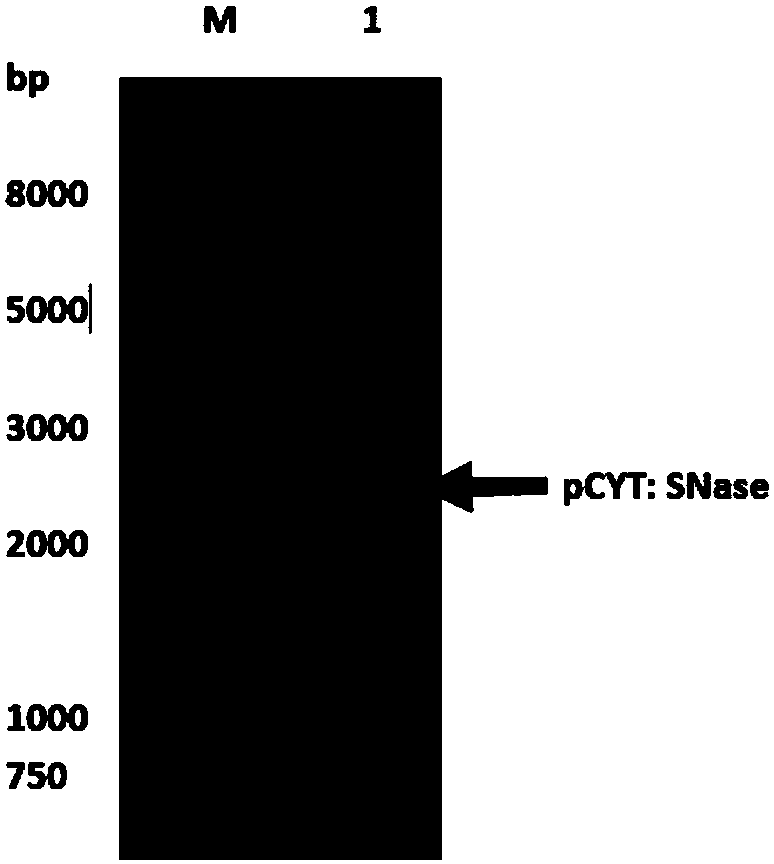
[0036] Example 1: Extraction of pCYT:SNase plasmid and verification by agarose gel electrophoresis
[0037] Use the plasmid mini-extraction kit (Beijing Tiangen Biochemical Technology Co., Ltd.) for plasmid extraction. The process is as follows: Add 500 μL of balance solution BL to the adsorption column CP3 (the adsorption column is placed in a collection tube), centrifuge at 12,000 rpm for 1 min, pour it out and collect For the waste liquid in the tube, put the adsorption column back into the collection tube; take 5mL of overnight cultured bacterial solution, add it to the centrifuge tube, centrifuge at 12000rpm for 1min, and suck out the supernatant as much as possible; add 250 μL of solution P1 (with RNaseA and lysozyme added in advance), use a pipette to thoroughly suspend the bacterial pellet, and place it in a 37°C water bath for 40 minutes; add 250 μL of solution P2 to the centrifuge tube, and gently turn it up and down 6-8 times Fully lyse the bacteria; add 350 μL of s...
Embodiment 2
[0038] Example 2: Induction of recombinant Lactococcus lactis strains L. lactis NZ9000 pCYT:SNase expresses SNase.
[0039] Lactococcus lactis L. lactis NZ9000 pCYT: SNase, according to the literature "Luis G. Bermúdez-Humarán et al. Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis [J]. FEMS Microbiology Letters, 224 (2003): 307-313" Prepared by the provided method, named pCYT:NUC in the literature. The recombinant lactic acid bacteria L. lactis NZ9000 pCYT: SNase, namely Lactococcus lactis NZ9000pCYT: SNase Lactococcus lactis NZ9000 pCYT:SNase was deposited in the China Center for Type Culture Collection, address: Wuhan, China, Wuhan University; the preservation number is CCTCC NO: M2016084, and the preservation time was March 4, 2016.
[0040] Lactococcus lactis L. lactis NZ9000 pCYT: SNase was inoculated in GM17 medium and cultured overnight at 30°C; the overnight culture solution was tran...
Embodiment 3
[0041] Embodiment 3: recombinant Lactococcus lactis L. lactis Pharmacodynamic evaluation of NZ9000 pCYT:SNase anti-type 1 diabetes vaccine.
[0042] Twenty-four 4-week-old female NOD / LtJ mice (purchased from Beijing Huafukang Biotechnology Co., Ltd., license number: SCXK (Beijing) 2012-0004) were randomly divided into two groups, respectively L. lactis NZ9000 group and L. lactis NZ9000pCYT: SNase group, 12 in every group, when the mice were five weeks old, they began to give oral administration once a day in the first week, and once a week from the second week after the administration until the mice were small. The administration was terminated when the mice were 20 weeks old. Before gavage, adjust the concentration of live bacteria in each group to 2×10 10 CFU / ml, the dosage is 4×10 9 CFU / 200μL / time / only.
[0043] (1) Recombinant Lactococcus lactis L. lactis Effect of NZ9000 pCYT:SNase Vaccine on Morbidity in NOD Mice.
[0044] From the beginning of the experime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com