Method for synthesizing modified aminosulfonate superplasticizer from wastewater produced in K acid production process
A sulfamic acid salt and production process technology, applied in chemical instruments and methods, heating water/sewage treatment, extraction water/sewage treatment, etc., can solve problems such as difficult to reuse, complex components, etc., to improve service life, The effect of low dosage and high concrete strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
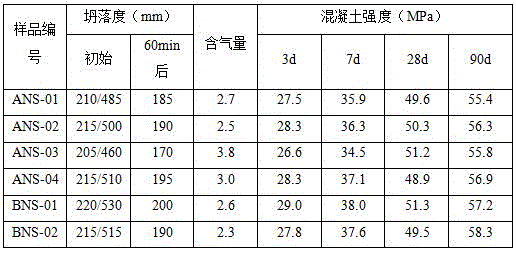
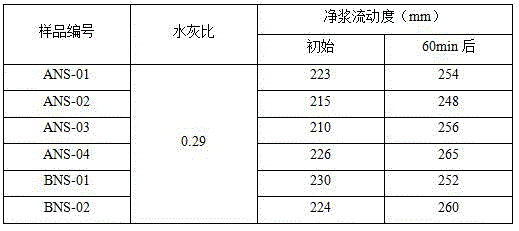
[0028] Put 30g of organic matter extracted from K-acid wastewater (including sodium sulfate content: 13.2%), 18g of sodium p-aminobenzenesulfonate and 32g of phenol into a four-necked reaction flask, add 185g of water, and heat up while stirring. When the temperature rises to 45 At ℃, use 32% ionic membrane NaOH solution to adjust the pH value of the solution to 8.8, continue to heat up to 70℃, and start to drop 26.5g of 37% formaldehyde at this temperature. After the dropwise addition of formaldehyde is completed, the temperature is raised to 98°C, and the reaction is maintained for 8 hours. After the end of the heat preservation reaction, the pH value of the material is adjusted to 11.2 with 32% ionic membrane NaOH solution, and the reaction is carried out at 98°C for 4 hours, and the reaction ends. Cool down to room temperature and unload to obtain the modified sulfamate superplasticizer, numbered ANS-01.
Embodiment 2
[0030] 22g of organic matter extracted in the K-acid wastewater (wherein sodium sulfate content: 9.6%), 15g of sodium sulfanilate and 40g of cresol are put into a four-necked reaction flask, add 175g of water, heat up while stirring, when the temperature rises to At 45°C, use 32% ionic membrane NaOH solution to adjust the pH of the solution to 8.3, continue to heat up to 70°C, and start adding 30.5g of 37% formaldehyde dropwise at this temperature. After the dropwise addition of formaldehyde is completed, the temperature is raised to 98°C, and the reaction is maintained for 10 hours. After the end of the heat preservation reaction, the pH value of the material is adjusted to 11.5 with 32% ionic membrane NaOH solution, and the reaction is carried out at 98°C for 3 hours, and the reaction is completed. Cool down to room temperature and unload to obtain the modified sulfamate superplasticizer, numbered ANS-02.
Embodiment 3
[0032] 13g of organic matter extracted from K acid wastewater (including sodium sulfate content: 5.8%), 16g of organic matter extracted from G salt wastewater (including potassium sulfate content: 8.6%), 17g of sodium p-aminobenzenesulfonate, 40g of bisphenol A and high Active lignin 5g is put into the four-necked reaction flask, add water 210g, heat up while stirring, when the temperature rises to 45°C, use 32% ionic membrane NaOH solution to adjust the pH value of the solution to 7.7, continue to heat up to 70°C, and At this temperature, 34.6 g of 37% formaldehyde was added dropwise. After the dropwise addition of formaldehyde is completed, the temperature is raised to 100°C, and the reaction is maintained for 9 hours. After the end of the heat preservation reaction, the pH value of the material is adjusted to 11.5 with 32% ionic membrane NaOH solution, and the reaction is carried out at 100°C for 5 hours, and the reaction ends. Cool down to room temperature and unload to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com