Cyclohexyl-hydrogen-peroxide-free cyclohexane oxidation liquid deacidification method for alkali liquid circulation
A technology of cyclohexane oxidation liquid and cyclohexane, which is applied in chemical instruments and methods, purification/separation of oxygenated compounds, preparation of organic compounds, etc., can solve the problem of consumption of NaOH, alkali cannot be closed and recycled, and the formation of large Alkali slag and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
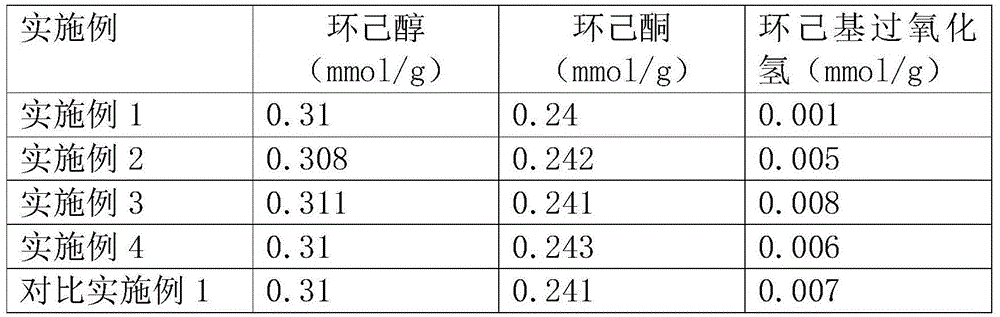
[0035] Add 50 g of cyclohexane oxidation solution without passing through and 1.22 g of 15% sodium carbonate aqueous solution into a 100 ml reaction flask, stir and neutralize at 60° C. for 5 minutes. The aqueous solution of the lower layer was separated at rest, and the pH value of the lower layer solution was detected to be 9.1. The solution was evaporated in an oil bath at 120° C. to a concentration of 55%, and then calcined at 600° C. in air for 1 hour to obtain 0.19 g of off-white powder.
[0036] The separated upper organic solution was analyzed by GC for the contents of cyclohexanol and cyclohexanone, and the results are shown in Table 1.
Embodiment 2
[0038] The white powder obtained in Example 1 was dissolved in 0.76 g of water to make a suspension with a concentration of 20%, and filtered with filter paper to obtain clarified lye. Add 50 grams of cyclohexane oxidation solution and the filtered lye sodium carbonate solution to the 100 ml reaction bottle, and stir and neutralize at 80° C. for 5 minutes. The aqueous solution of the lower layer was separated at rest, and the pH value of the lower layer solution was detected to be 9.5. The solution was evaporated in an oil bath at 120° C. to a concentration of 50%, and then calcined at 700° C. in air for 1 hour to obtain 0.20 g of off-white powder.
[0039] The separated upper organic solution was analyzed by GC for the contents of cyclohexanol and cyclohexanone, and the results are shown in Table 1.
Embodiment 3
[0041] The white powder obtained in Example 2 was dissolved in 0.80 g of water to make a suspension with a concentration of 20%, and filtered with filter paper to obtain clarified lye. Add 50 grams of cyclohexane oxidation solution and the filtered lye sodium carbonate solution to the 100 ml reaction bottle, and stir and neutralize at 80° C. for 5 minutes. The aqueous solution of the lower layer was separated at rest, and the pH value of the lower layer solution was detected to be 9.5. The solution was evaporated in an oil bath at 120° C. to a concentration of 50%, and then calcined at 800° C. in air for 1 hour to obtain 0.21 g of off-white powder.
[0042] The separated upper organic solution was analyzed by GC for the contents of cyclohexanol and cyclohexanone, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com