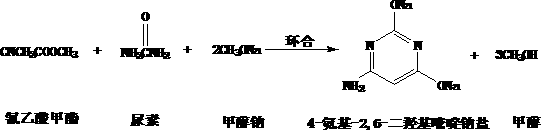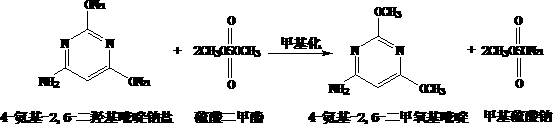A kind of preparation method of 4-amino-2,6-dimethoxypyrimidine
A technology of dimethoxypyrimidine and hydroxypyrimidine sodium salt is applied in the field of preparation of 4-amino-2,6-dimethoxypyrimidine, and can solve the problems of hidden dangers in production safety, aggravated by-products, high pressure on environmental protection and the like, Achieve the effect of reducing equipment requirements, simple equipment, and reducing pollutant emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0008] 1. The process is simple and the equipment is simple. The acidification and chlorination processes in the traditional process are omitted, and the finished product can be obtained only in two steps of cyclization and methylation. And all reactions can be carried out in the same vessel, reducing equipment requirements. 2. The yield has been greatly improved. Because 4-amino-2,6-dihydroxypyrimidine sodium salt is easy to open and hydrolyze in water, the yield of the original process is not high. The 4-amino-2,6-dihydroxypyrimidine sodium salt generated by the present invention does not need to be separated, and can directly participate in the next step reaction, thereby avoiding its ring-opening hydrolysis. 3. The chlorination process is omitted, the production cost is reduced, and the operating environment is greatly improved. 4. Significantly reduce pollutant emissions. The acidification, chlorination and methoxylation processes in the original process all produce a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com