A kind of synthetic method of 5 azaindole
An azaindole and a synthesis method technology are applied in the field of 5-azaindole synthesis, can solve the problems of high synthesis cost, long reaction time, low reaction efficiency and the like, and achieve lower synthesis cost, high controllability and optimization. The effect of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
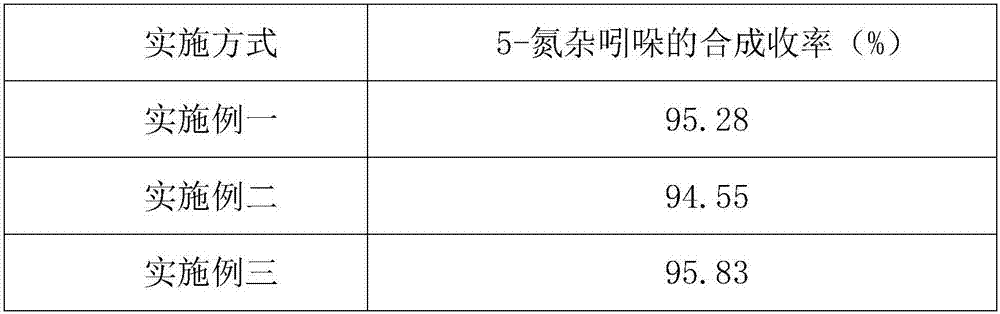
Embodiment 1
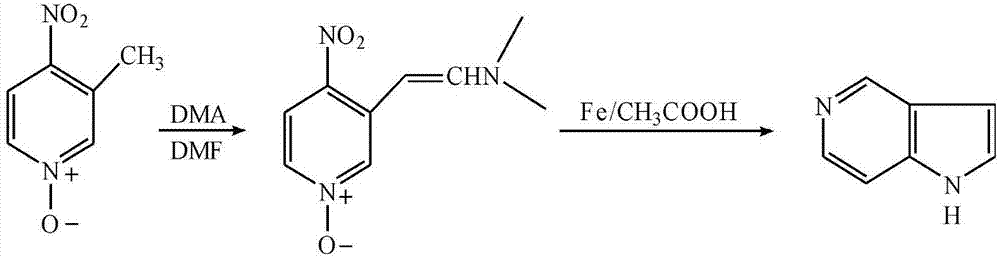
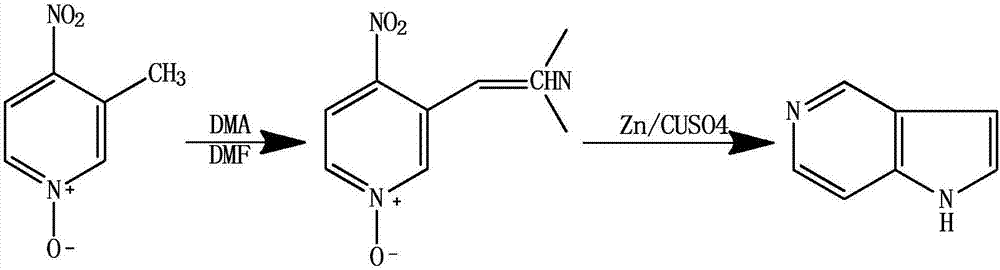
[0040] A kind of synthetic method of 5-azaindole, take 3-methyl-4-nitropyridine oxynitride as starting raw material, first generate enamine, then undergo reduction reaction, generate 5-azaindole, comprise the following Steps:
[0041] Step 1: Add 3.08g of 3-methyl-4-nitropyridine nitrogen oxide, 12mL of DMF, and 10mL of DMA into a 100mL round-bottomed flask in sequence, place in an oil bath, heat up to 110°C, and reflux for 20 minutes; After the reaction solution was cooled, the solvent was distilled off under reduced pressure, 10 mL of absolute ethanol was added, filtered with suction, and the solid was washed and dried to obtain 3-dimethylaminovinyl-4-nitropyridine nitrogen oxide;
[0042] The second step: add 3.00g of 3-dimethylaminovinyl-4-nitropyridine nitrogen oxide and 30mL of copper sulfate aqueous solution (mass concentration is 5%) in a 100mL pear-shaped flask, and add 1g of zinc powder under stirring at room temperature ; The temperature was raised to 50°C, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com