Reactive flame retardant applied to polyurethane materials and preparation method of reactive flame retardant
A technology of reactive flame retardants and polyurethane materials, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve limited flame retardant effects, single flame retardant elements, and synergistic effects No way to play and other problems, to achieve a good flame retardant effect, the effect of a simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] A preparation method of a reactive flame retardant applied to polyurethane materials, the specific steps are as follows:
[0021] (1) Add reactant tetrabromophthalic anhydride, N,N-di(2-hydroxyethyl)aminomethylenephosphonic acid diethyl ester (FRC-6) and solvent into the reaction vessel; stir until tetrabromophthalic anhydride is completely dissolved , heated to 60-120°C under stirring conditions, and reacted for 2-12 hours to synthesize tetrabromophthalic acid (2-hydroxyethylphosphonic acid diethyl methylene) aminomono-2-ethyl ester;
[0022] (2) After the reaction, cool down to below 50°C, filter, and distill to recover the solvent to obtain viscous tetrabromophthalic acid (2-hydroxyethylphosphonic acid diethyl methylene) aminomono-2 - Ethyl ester products.
[0023] The reactant tetrabromophthalic anhydride:diethyl N,N-bis(2-hydroxyethyl)aminomethylenephosphonate (FRC-6) has a mass ratio of 3.6:1-0.9:1.
[0024] The solvent is one of benzene, toluene, xylene, cycloh...
Embodiment 1
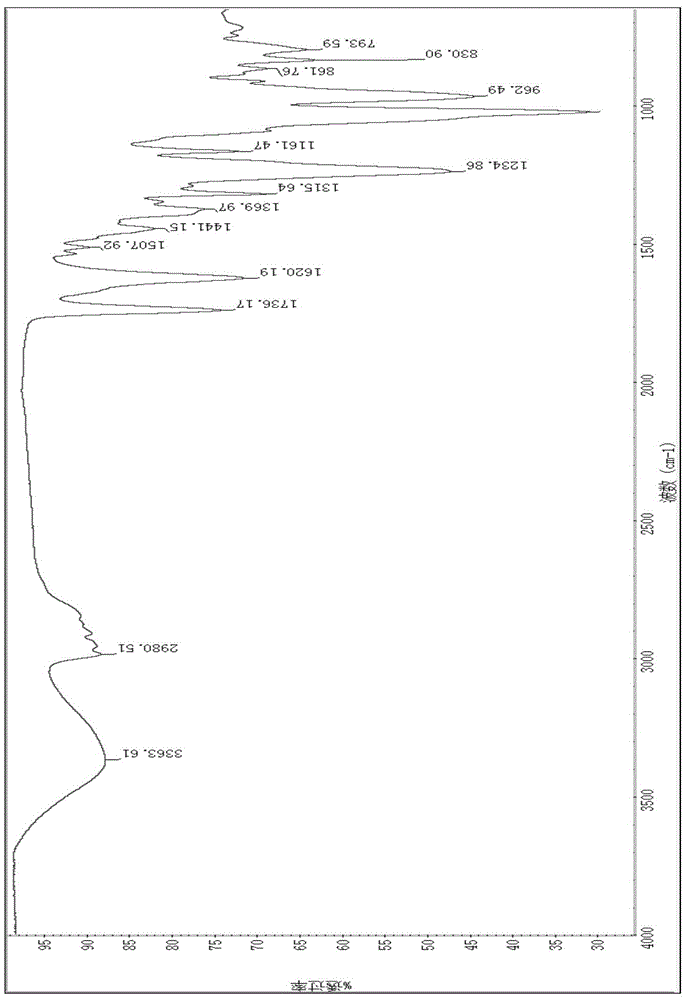
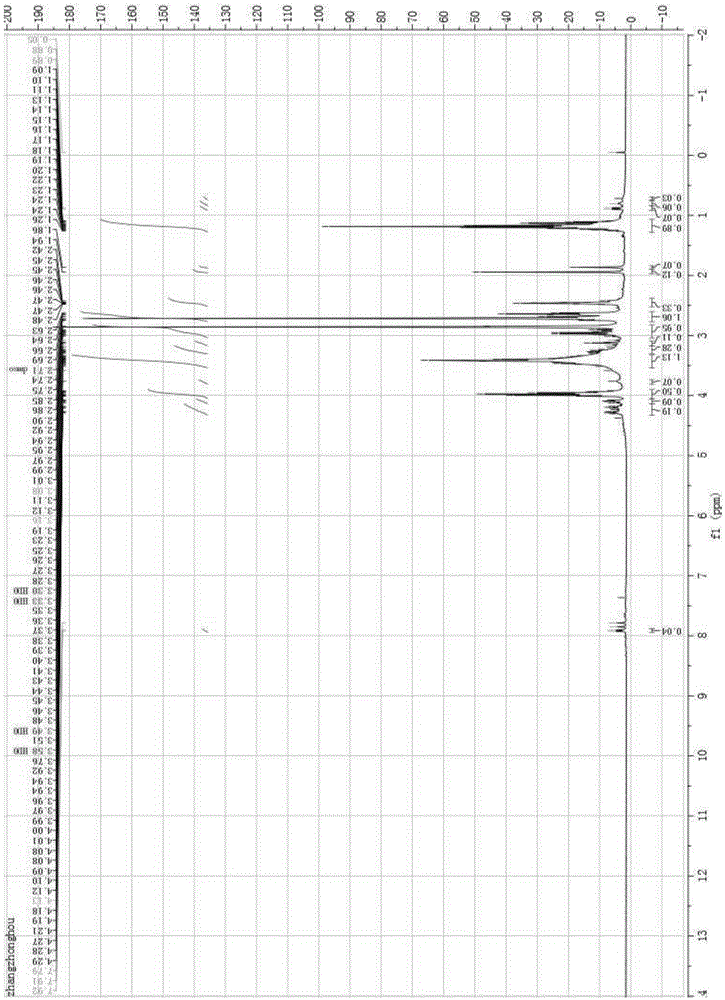
[0027] Add 100g of tetrabromophthalic anhydride, 50g of FRC-6 and 50mL of benzene in sequence to a 500mL four-neck flask equipped with a stirrer, condenser and thermometer, start stirring until the tetrabromophthalic anhydride is completely dissolved, raise the temperature to 80°C, and react for 10 hours at a constant temperature . Cool down to 20°C, filter, and recover benzene by distillation to obtain a viscous TBPAHM product, which is detected by a NICOLETIS10 Fourier transform infrared spectrometer from Thermo Fisher. The FTIR of this product is as follows: figure 1 shown. The functional group vibrations corresponding to the main absorption peaks in the infrared spectrum are: 3364cm -1 (v-OH), 2981cm -1 (VS-CH3), 2906cm -1 (V-P-CH2), 1736(V-C=O)cm -1 , 1620cm -1 、1532cm -1 、1508cm -1 (Phenyl ring hexasubstituted), 1316cm -1 (V-CH3), 1370cm -1 (V-P=O), 1235cm -1 (V-C-O), 1162cm -1 (V-C-N), 1017cm -1 (V-P-O), 627cm -1 (V-C-Br). Adopt the Agilent400MR nuclear ma...
Embodiment 2
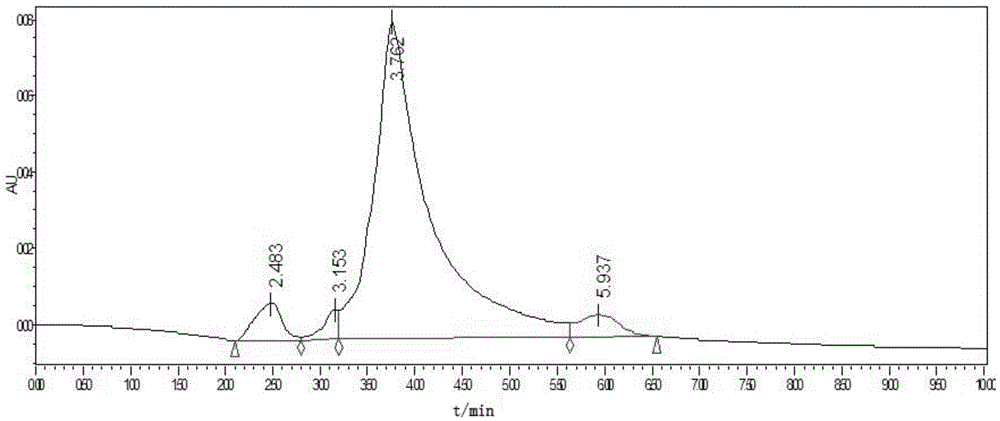
[0029] Add 90g of tetrabromophthalic anhydride, 60g of FRC-6 and 100mL of toluene in sequence to a 500mL four-necked flask equipped with a stirrer, condenser and thermometer, start stirring until the tetrabromophthalic anhydride is completely dissolved, raise the temperature to 95°C, and react for 8 hours at a constant temperature . Cool down to 25°C, filter, and recover toluene by distillation to obtain a viscous TBPAHM product. The content of TBPAHM is 88.3%. It is detected by the LC-1525 high-performance liquid chromatograph of Waters Corporation in the United States. The high-performance liquid chromatogram of the product is Such as image 3 shown.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap