Inducible expression, purification and activity identification method of restructured lunasin polypeptide in pichia pastoris
A technology of inducing expression and Pichia pastoris, applied in the field of genetic engineering, can solve the problems of low yield, cumbersome Lunasin steps, restricting research and application, etc., and achieves the effects of high yield, simple discrete purification steps, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Construction of recombinant expression plasmid
[0050] The secretory Pichia pastoris expression plasmid pPIC9K (commercially available) was selected as the target gene expression vector, and the Lunasin sequence with EcoRI and NotI restriction sites was designed as GAATTCTCCAAATGGCAGCACCAGCAAGACAGCTGCCGCAAGCAGCTCCAGGGGGTGAACCTCACGCCTTGCGAGAAGCACATCATGGAGAAGATCCAAGGCCGCGGCGATGACGATGATGATGATGACGACGACGACGCGGCCCTACTACTGCTGCTGCGCCGG.
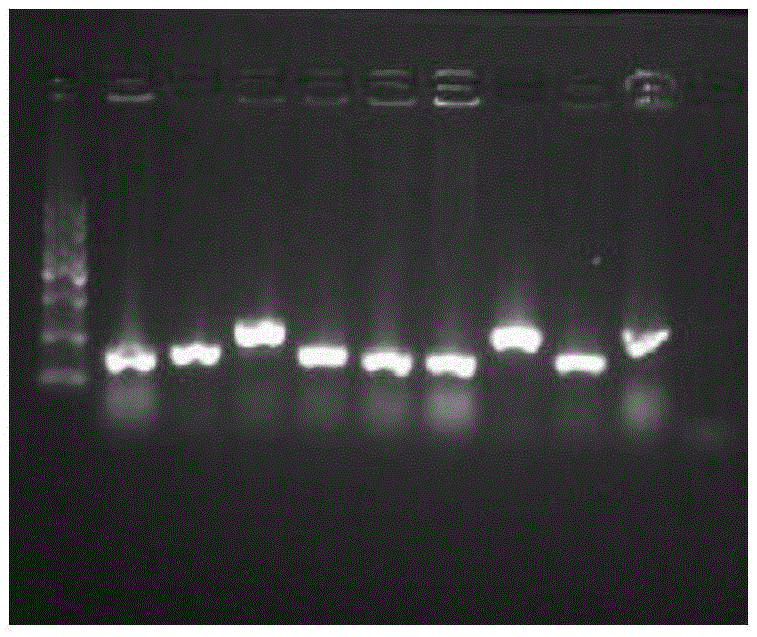
[0051] Design specific amplification primers: upstream primer: 5'-GGAATTCTCCAAATGGCAGCAC-3'; downstream primer: 5'-TTGGCCGCGTCGTCGTCATCATC-3'. PCR amplification was performed to obtain the target gene fragment. PCR conditions were: 94°C for 5 min, 94°C for 30s, 55°C for 30s, 72°C for 60s, 35 cycles, and 72°C for 10 min.
[0052] Using the restriction enzymes EcoRI and NotI double digestion, the plasmid pPIC9K double-digested with the same restriction enzymes EcoRI and NotI was ligated with the PCR product after digestion with T4 DNA liga...
Embodiment 2
[0070] Example 2 Identification of Physiological Activity of Recombinant Lunasin Polypeptide
[0071] 3T3-L1 preadipocytes were seeded in a 12-well plate at a density of 10,000 cells / mL, and after growth and fusion, they were cultured for another 48 hours, and induction solution I was added. The induction solution I contained 10% FBS, 1% double antibody, 10 μg / ml insulin, 0.5mM IBMX (3-isobutyl-1-methylxanthine) and DMEM medium of 0.1 μM DEX (dexamethasone); after culturing for 48h, it was replaced with induction solution II, which was composed of Cultured in DMEM medium of 5 μg / ml insulin; after culturing for 48 hours, it was replaced with DMEM medium containing 50 μg / ml (recombinant lunasin group-50) or 100 μg / ml (recombinant lunasin group-100) recombinant Lunasin polypeptide, and added with 5 μg / ml Rosiglitazone was used as a positive control group, and no lunasin was added as a blank control group. After culturing for 48 hours, the mice were fixed in 10% formaldehyde buff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com