Preparation method of maleic acid asenapine
A technology of asenapine maleate and asenapine maleate is applied in the research field of synthesis technology of new atypical antipsychotic drug asenapine maleate, which can solve the problems of low yield, Problems such as poor product quality and no production value can achieve the effect of improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
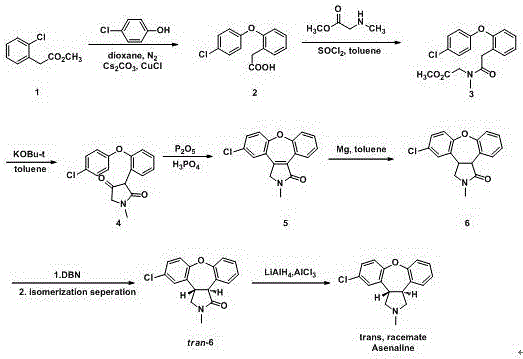
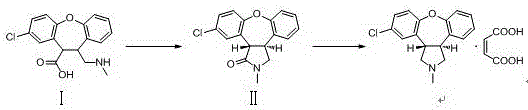
[0025] Example 1: (3aS,12bS)-11-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxazin[4,5 -c] Preparation of pyrrol-1-one (compound II).
[0026] Add 35.4g of compound Ⅰ (0.1mol), 12.3g of sodium acetate (0.15mol), 25.2g of acetic acid (0.42mol), and 350ml of absolute ethanol into a 500ml three-necked flask, reflux and stir, and react for 4 hours. TLC spotting test shows that the reaction is complete . Atmospheric pressure distillation removed 175 ml of the solvent, and the temperature was naturally lowered to obtain a cloudy ethanol solution of compound II. Add 175ml of water, continue to cool down, keep warm at 5°C for 3h, filter under reduced pressure, and wash the filter cake with a lot of water to obtain 28.4g of light yellow solid, yield: 95.0%. MS (+1): 300.1. 1 HNMR: δ (ppm, DMSO), 7.790 (dd, 1H), 7.139-7.784 (m, 6H), 4.059-4.089 (m, 1H), 3.865-3.885 (m, 1H), 3.524-3.849 (m, 2H) ), 2.894(s,3H).
Embodiment 2
[0027] Implementation example two: the preparation of asenapine maleate
[0028] Add 10g of compound II (33.43mmol) and 100ml of dichloromethane into a 500ml three-neck flask, at 0-5°C, under nitrogen protection for 10-15 minutes, add 5.1ml of triethylchlorosilane (40.12mmol), and continue stirring for 10- After 15 minutes, 19.5ml (46.81mmol) of lithium aluminum hydride tetrahydrofuran solution was added dropwise at 0-10°C, stirred at room temperature for 12h, and detected by TLC until the reaction was complete. 30ml of 2M sodium hydroxide solution was slowly quenched, extracted with 25ml of dichloromethane, collected the organic phase, washed with 20% sodium chloride solution, and distilled under reduced pressure to obtain the residue, dissolved in 50ml of isopropanol, and added 5.0g of maleic acid ( 43.46mmol) stirred at room temperature for 2-3h, lowered to 0-10°C and continued to stir for 2-3h. Filtered under reduced pressure, washed with 10ml of isopropanol, dried at 50-5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com