Hot-melt granulation method of pharmaceutical adjuvant of controlled/slow-released agent
A technology of pharmaceutical excipients and sustained-release agents, applied in the field of carbomer hot-melt granulation, can solve problems such as poor fluidity, small particle size, and strong electrostatic interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
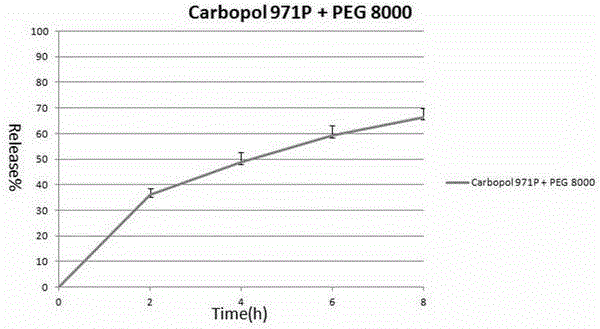
[0022] 120 grams of Carbopol 971P was placed in a pot of a wet granulator, and Carbopol 971P was heated with an 85 degree water bath through the interlayer of the pot. Under the stirring of a stirring paddle at 50 rpm, Carbopol971P will gradually heat up. When the temperature reaches 65-70 degrees, 120 grams of polyethylene glycol 8000 (PEG8000) particles with an average particle size of 100 μm are slowly poured into the wet granulator. inside the pot, then increase the paddle speed to 60 rpm and keep the water bath heated at 85 degrees. After about 10-20 minutes, the carbomer will evenly adhere to the surface of the polyethylene glycol 8000 wax particles, forming denser particles. After the granulation is completed, the material is poured out, slightly cooled, and passed through a 20-mesh sieve, which is the final product with an average particle size of 72 μm.
[0023] 32.9 grams of theophylline anhydrous, 50 grams of Carbopol971P and PEG8000 above-mentioned granular produc...
Embodiment 2
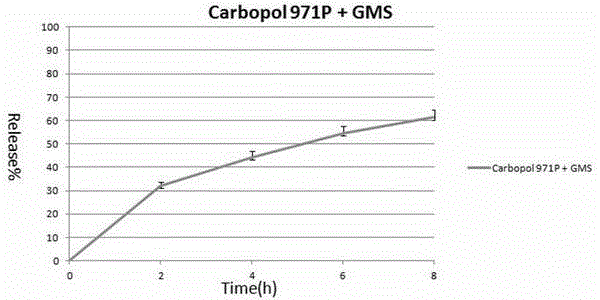
[0025] 120 grams of Carbopol 971P was placed in a pot of a wet granulator, and Carbopol 971P was heated with an 80-degree water bath through the interlayer of the pot. Under the stirring of a stirring paddle at 50 rpm, Carbopol971P will gradually heat up. When the temperature reaches 60-65 degrees, 120 grams of glycerol monostearate (GMS) particles with an average particle size of 100 μm are slowly poured into wet granulation. Then increase the speed of the stirring paddle to 60 rpm and keep the water bath heated at 80 degrees. After about 10-20 minutes, the carbomer will evenly adhere to the surface of the glycerol monostearate wax particles to form denser particles. After the granulation is completed, the material is poured out, cooled a little, and passed through a 20-mesh sieve, which is the final product. Its average particle size is 69 μm.
[0026] Mix 32.9 grams of anhydrous theophylline, 50 grams of Carbopol 971P and glycerol monostearate into granules, 16.6 grams of...
Embodiment 3
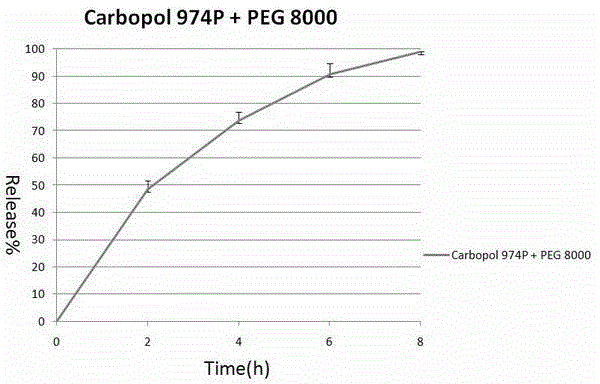
[0028] 120 grams of Carbopol 974P was placed in a pot of a wet granulator, and Carbopol 974P was heated with an 85 degree water bath through the interlayer of the pot. Under the stirring of the stirring paddle at 50 rpm, the temperature of Carbopol974P will gradually increase. When the temperature reaches 65-70 degrees, 120 grams of polyethylene glycol 8000 (PEG8000) particles with an average particle size of 100 μm are slowly poured into the wet granulator. inside the pot, then increase the paddle speed to 60 rpm and keep the water bath heated at 85 degrees. After about 10-20 minutes, the carbomer will evenly adhere to the surface of the polyethylene glycol 8000 wax particles, forming denser particles. After the granulation is completed, the material is poured out, cooled a little, and passed through a 20-mesh sieve, which is the final product. Its average particle size is 78 μm.
[0029] 32.9 grams of theophylline anhydrous, 50 grams of Carbopol974P and the above-mentioned...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com