A kind of method for preparing immobilized aromatase
An aromatase, immobilized enzyme technology, applied in biochemical equipment and methods, immobilized on or in inorganic carriers, oxidoreductase, etc. Evaluate problems such as drugs to be screened, and achieve high research value and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
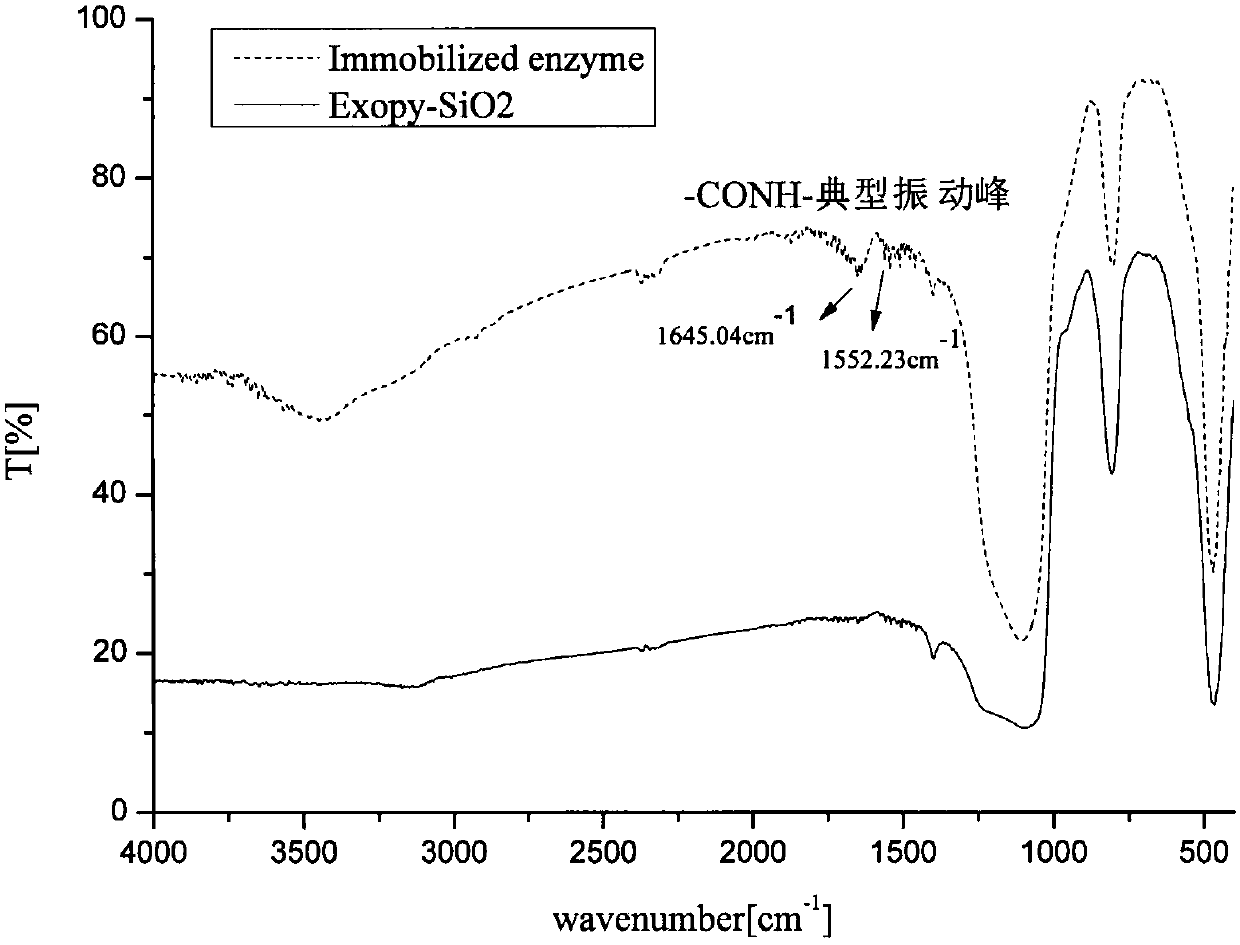
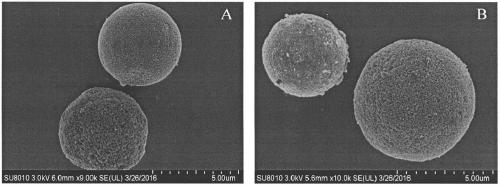
[0031] (1) Weigh 4g of mesoporous silicon spheres (sepax@HP-silica; 5μm, ), add 100mL of 10% hydrochloric acid, reflux at 90°C for 8 hours with stirring, then wash with a large amount of deionized water until neutral, and vacuum dry at 150°C. An activated mesoporous silica sphere carrier is obtained.
[0032] (2) Weigh 500 mg of the activated mesoporous silicon spheres obtained in step (1) and disperse them in 20 mL of anhydrous toluene, add 250 μL of γ-glycidyl etheroxypropyltrimethoxysilane (KH-560) dropwise, and stir at 90 °C Refluxed for 12 h, then washed three times with toluene and acetone, dried in vacuo at 60 °C, and stored at 4 °C to obtain epoxy-modified functionalized mesoporous silicon spheres (Exopy-SiO 2 ).
[0033] (3) Preparation of 100mmol / L potassium phosphate buffer (pH7.4): Weigh 4.56g K 2 HPO 4 Dissolve in 200mL deionized water to make liquid A, weigh 2.72g KH 2 PO 4 Dissolve in 200mL deionized water to form B liquid, and mix A and B at (A:B) 80.2:1...
Embodiment 2
[0041] (1) Prepare the enzyme solution for immobilization according to step (5) of Example 1, with a concentration of 10 pmol / mL.
[0042] (2) Weigh 5 mg of the epoxy-modified functionalized mesoporous silica spheres obtained in step (2) of Example 1 and wash them three times with 0.5 mL of potassium phosphate buffer (100 mmol / L, pH 7.4), then add step (1) The prepared enzyme solution was 0.5mL, rotated and shaken at 4°C for more than 30min, centrifuged at low temperature and high speed, and the precipitate was washed 3 times with 0.5mL potassium phosphate buffer (100mmol / L, pH7.4), and the enzyme activity was measured.
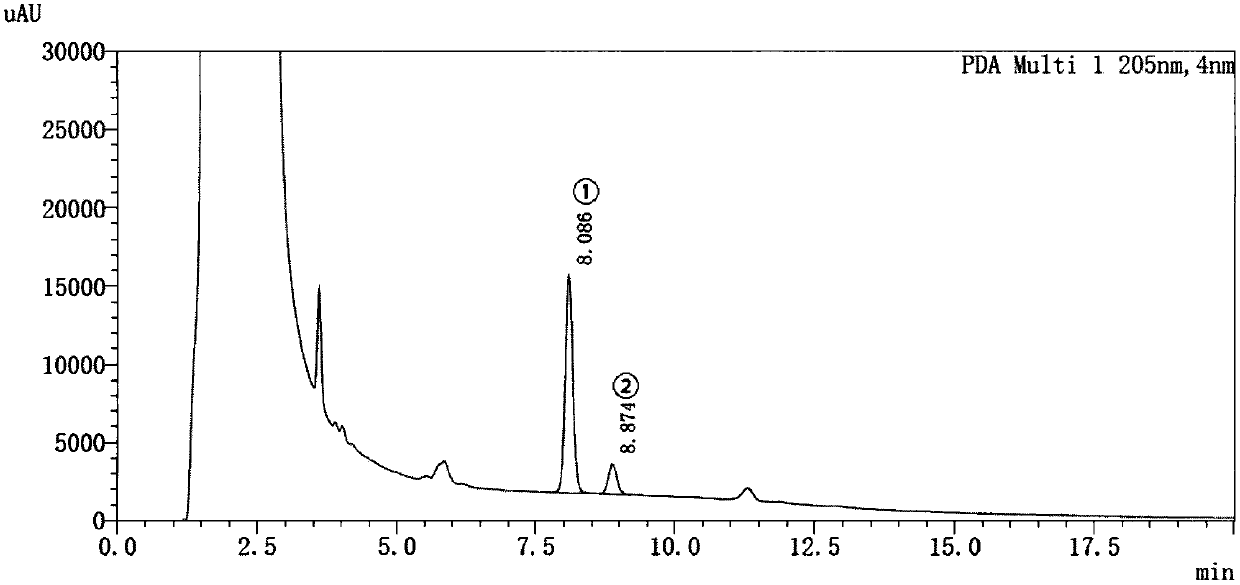
[0043] (3) The relationship between the enzymatic reaction of free aromatase and time: 170 μL potassium phosphate buffer solution (100 mmol / L, pH 7.4), 5 μL androstenedione working solution, 5 pmol aromatase, After vortexing to mix well, add 20 μL NADPH to start the reaction after 5 minutes in a water bath at 37°C, and react each tube in a water bath for 10 m...
Embodiment 3
[0047] (1) According to step (1) (2) of embodiment 1, functionalized mesoporous silicon spheres modified by epoxy group are obtained;
[0048] (2) Preparation of 100mmol / l potassium phosphate buffer solution (pH7.4): weigh 4.56g K 2 HPO 4 Dissolve in 200mL deionized water to make liquid A, weigh 2.72g KH 2 PO 4 Dissolve in 200mL deionized water to form B liquid, and mix A and B at (A:B) 80.2:19.8.
[0049] (3) Preparation of androstenedione stock solution and working solution: Weigh 2 mg androstenedione into a 1 mL volumetric flask, dilute to the mark with DMSO, and store at -20°C, which is a 2 mg / mL stock solution; take 5 μL The stock solution was mixed with 95 μL of potassium phosphate buffer (100 mmol / l, pH 7.4) to obtain a 100 μg / mL androstenedione working solution.
[0050] (4) Preparation of enzyme solution for immobilization: Aromatase from the baculovirus-insect cell expression system (Corning, USA) was diluted to 30 pmol / mL with potassium phosphate buffered saline...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic strength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com