Method of using surface-carboxyl-modified magnetic spheres to prepare immobilized enzyme to screen aromatase inhibitors
A technology of aromatase and carboxyl modification, applied to biochemical equipment and methods, oxidoreductases immobilized on or in inorganic carriers, etc., can solve problems such as relatively high cost, medical ethics issues, and substrate competition. Achieve the effects of improved storage stability, high research value, fast and convenient recycling and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
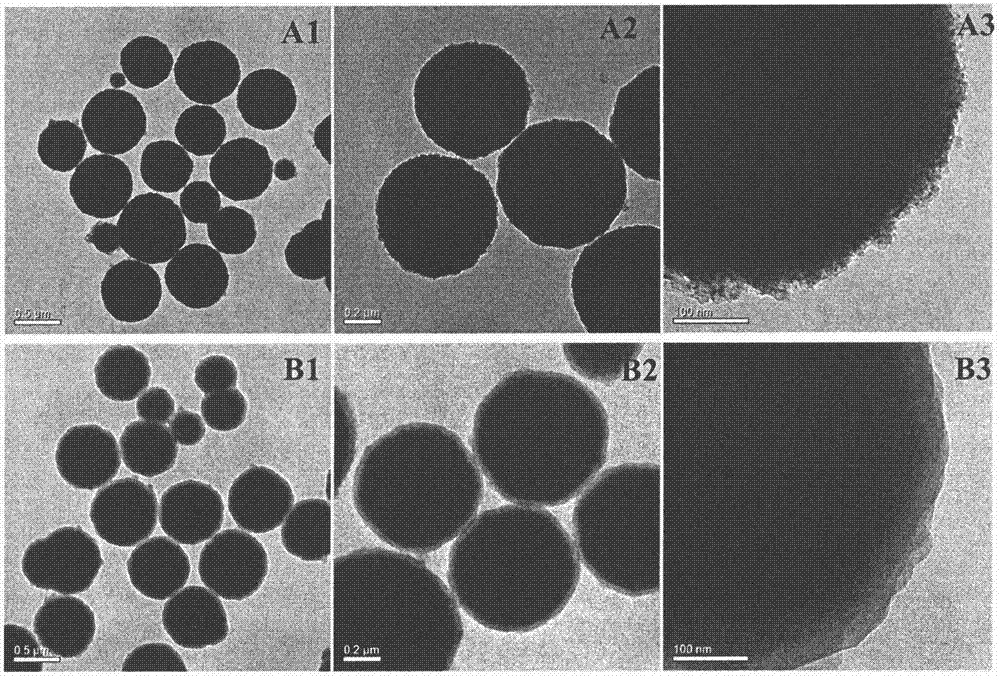
[0034] Example 1 Preparation of Surface Carboxyl Modified Magnetic Ball Carrier
[0035] (1) Fe 3 o 4 Preparation of magnetic core: Weigh a certain amount of ferric chloride (FeCl 3 ·6H 2O), trisodium citrate, and ammonium acetate were placed in a dry round bottom flask. Add a certain amount of ethylene glycol, stir to fully dissolve. Heat and stir in an oil bath at 170-200°C for 1-2h. After the solution was slightly cooled, the black reaction solution was poured into a high-pressure reactor with polytetrafluoroethylene, and reacted in a sealed oven at 200° C. for 8-16 hours. Then take out the reaction kettle, cool it down to room temperature naturally, pour the magnetic ball solution that has finished the reaction into a beaker for magnetic sedimentation, and after obvious solid-liquid separation occurs, discard the supernatant, and dehydrate the solid magnetic balls with absolute ethanol. Washed three times with deionized water. Then put it into a vacuum drying oven a...
Embodiment 2
[0040] Example 2 Preparation of surface carboxyl-modified magnetic sphere immobilized aromatase
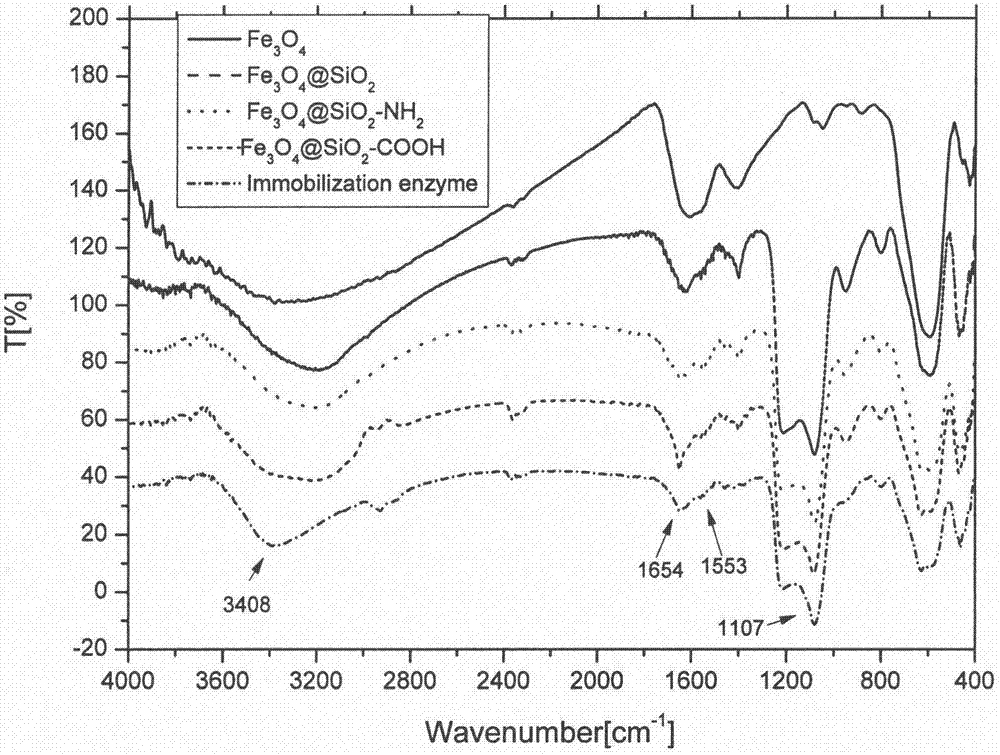
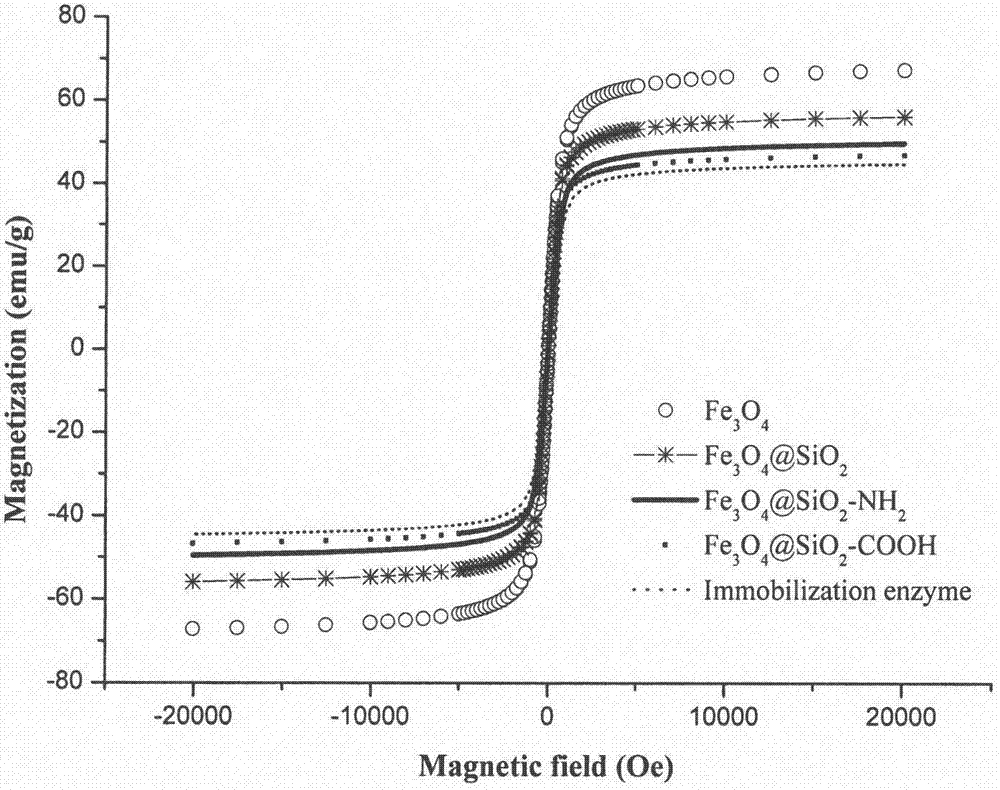
[0041] (1) According to Example 1, surface carboxyl-modified magnetic spheres (Fe 3 o 4 @SiO 2 -COOH).
[0042] (2) Preparation of potassium phosphate buffer (100mmol / L, pH 7.4): Weigh 4.56g K 2 HPO 4 Dissolve in 200mL deionized water to make liquid A, weigh 2.72g KH 2 PO 4 Dissolve in 200mL deionized water to form B solution, and mix A and B at a ratio of 80.2:19.8.
[0043] (3) Preparation of 0.1mol / L 2-(N-morpholine)ethanesulfonic acid monohydrate (MES) buffer solution: Weigh 1.9524g MES and dissolve it in 100mL deionized water, adjust the pH to 4.0-7.0 with 50% NaOH, Store at 4°C for later use.
[0044] (4) Weigh 7.68mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) and 11.5mg N-hydroxysuccinimide (NHS) in 5mL Add 2 mL of 0.1 mol / L MES buffer solution to the EP tube, vortex and mix well, and use it as an activation solution.
[0045] (5) take b...
Embodiment 3
[0047] Example 3 Determination of Surface Carboxyl Modified Magnetic Ball Immobilized Enzyme Enzyme Activity
[0048] (2) Preparation of nicotinamide adenine dinucleotide phosphate (NADPH) working solution: Weigh 8.33mg of NADPH (BIOSHARP company) into a 1mL volumetric flask, dilute with potassium phosphate buffer (100mmol / L, pH 7.4) To the mark, mix well, and store in a refrigerator at 4°C.
[0049] (3) The method for measuring the activity of the immobilized enzyme is as follows: draw 20 μL of the immobilized enzyme prepared in step (5) of Example 2 into a 2mLEP tube, and after magnetically absorbing the supernatant, add 175 μL of potassium phosphate buffer (100 mmol / L , pH 7.4), 5 μL androstenedione working solution, mix well, after shaking at 37°C for 5 minutes, add 20 μL NADPH working solution to start the reaction, react for 20 minutes, then collect the supernatant by magnetic force, take 50 μL of the supernatant and inject it into the high performance liquid phase spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com