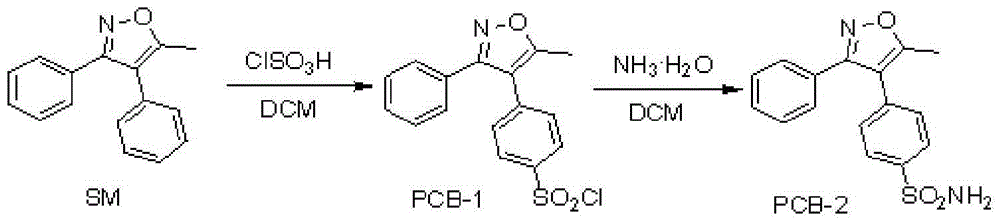
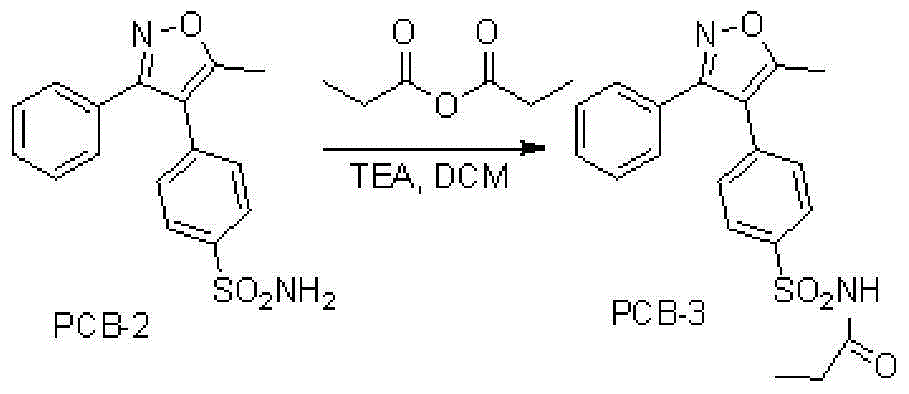
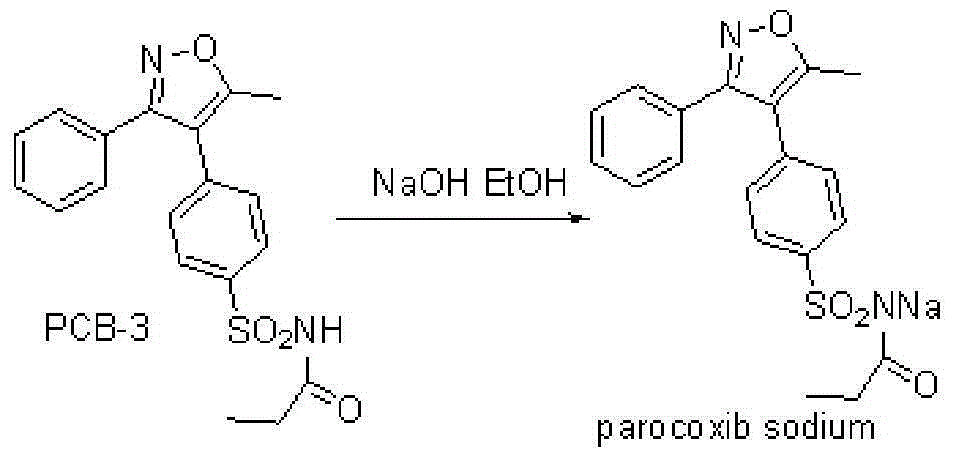
Parecoxib sodium freeze-dried powder, preparation method and powder product thereof
A technology of parecoxib sodium and freeze-dried powder, which is applied in the direction of freeze-dried transportation, medical preparations of non-active ingredients, anti-inflammatory agents, etc., can solve the problems of complex powder components and complicated preparation process, and achieve variety and use. The effect of small amount, simple preparation method and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Add about 160ml of water for injection into the beaker and control the water temperature below 40°C, add 2.12g of parecoxib sodium, stir until completely dissolved, and add water for injection to a total of 200ml. Use sodium hydroxide solution to adjust the pH value of the solution to 8.5, stir evenly, and filter through a microporous membrane. After the filtrate is inspected for visible foreign matter, it is divided into 5ml borosilicate tube glass injection bottles, each bottle of 1ml solution, and Based on parecoxib, each bottle contains 10mg / 1ml of parecoxib. After the packaging is completed, the rubber stopper is half-pressed, and the product is sent to a freeze-drying box for freeze-drying. Control the temperature of the product from room temperature to -40°C within 2 hours and keep it warm for 3 hours. Then evacuate to a vacuum degree of about 25 Pa, and control the temperature to rise to 0°C for 12 hours, then control the temperature to rise to 40°C for 5 hours...
Embodiment 2
[0075] Add about 160ml of water for injection into the beaker and control the water temperature below 40°C, add 4.24g of parecoxib sodium, stir until completely dissolved, add water for injection to a total of 200ml, add sodium hydroxide solution to adjust the pH of the solution to 8.5. Stir evenly and filter through a microporous membrane. After passing the inspection of visible foreign matter, the filtrate is divided into 5ml borosilicate tube glass injection bottles. Each bottle has 1ml solution, and each bottle contains Parecoxib. Coxib 20mg / 1ml. After the packaging is completed, the rubber stopper is half-pressed, and the product is sent to a freeze-drying box for freeze-drying. Control the temperature of the product from room temperature to -40°C within 2 hours and keep it warm for 3 hours. Then evacuate to a vacuum degree of about 25 Pa, and control the temperature to rise to 0° C. over 12 hours, then control the temperature to rise to 40° C. over 5 hours, and keep the...
Embodiment 3
[0078] Add about 160ml of water for injection into the beaker and control the water temperature below 40°C, add 4.24g of parecoxib sodium, stir until it dissolves completely, add water for injection to a total of 200ml, add sodium hydroxide solution to adjust the pH value of the solution to 9.0, Stir evenly and filter through a microporous membrane. After passing the inspection of visible foreign matter, the filtrate is divided into 7ml borosilicate tube glass injection bottles. Each bottle contains 2ml solution, and each bottle contains Parecoxib Coxib 40mg / 2ml. After the packaging is completed, the rubber stopper is half-pressed, and the product is sent to a freeze-drying box for freeze-drying. Control the temperature of the product from room temperature to -45°C within 2 hours and keep it warm for 3 hours. Then evacuate to a vacuum degree of about 10 Pa, and control the temperature to rise to 0° C. in 12 hours, then control the temperature to rise to 40° C. in 5 hours, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com