Use of diarylheptanoid compound in preparation of drug or food for preventing and treating psoriasis
A technology of diphenylheptane and compounds, applied in the field of application of diphenylheptane compounds in the preparation of medicines or food for the prevention and treatment of psoriasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
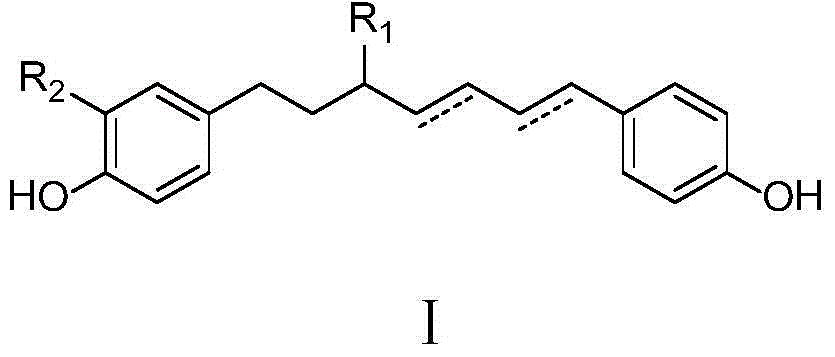
[0029] Example 1: Extraction and preparation of diphenylheptane compounds of the present invention
[0030] Take dried Guangxi zedoary turmeric rhizomes and extract them with 95% ethanol (ethanol: water 95:5, v / v). The extracts are separated by HP-20 macroporous adsorption resin column chromatography, followed by water, 30% ethanol, 90-100% Ethanol gradient elution (12L for each gradient eluate), collect each eluate, and concentrate under reduced pressure to obtain water elution part, 30v / v% ethanol elution part, and 90-100v / v% ethanol elution Part: Take the 90~100v / v% ethanol elution part, separate it by silica gel column chromatography, use chloroform-methanol gradient elution (each gradient eluent volume is 12L), collect the fraction E with the chloroform-methanol ratio of 9:1 , Fraction E was separated by ODS column chromatography, eluted with a methanol-water gradient, and fraction E-5 with a methanol-water ratio of 6:4 was collected. Fraction E-5 was separated by preparativ...
Embodiment 2
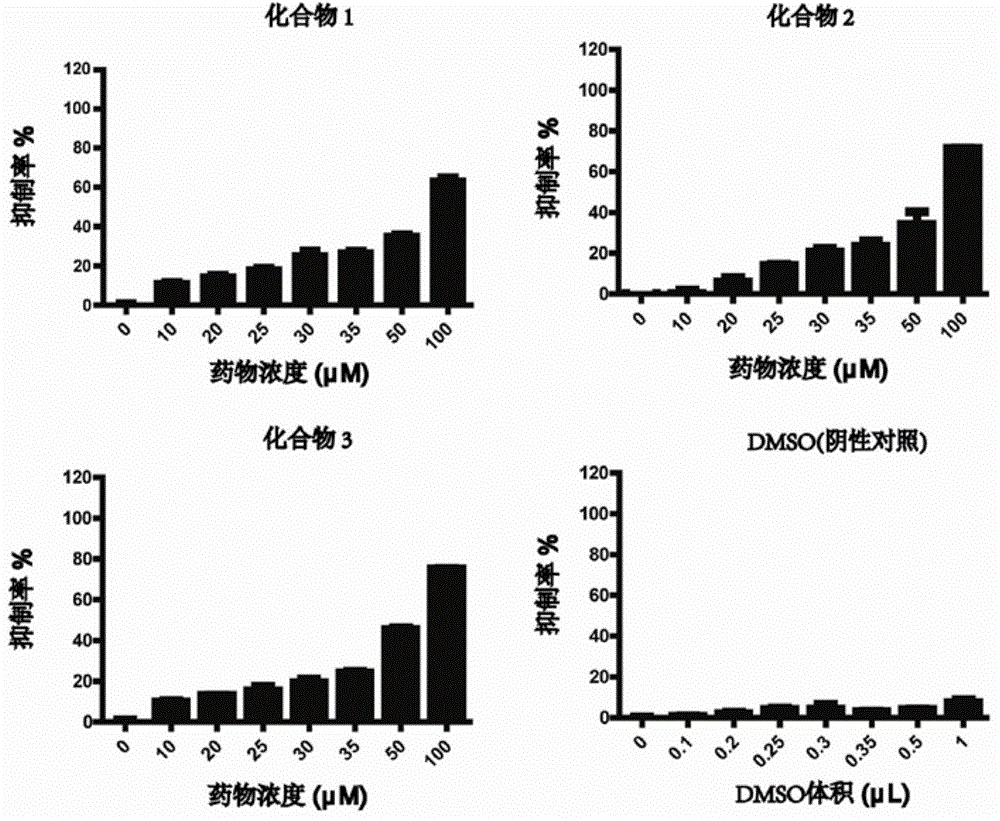
[0031] Example 2: Detection of the diphenylheptane compound of the present invention inhibiting the proliferation of human T cell lymphoma cell line HH cells in vitro
[0032] Collect HH cells in logarithmic growth phase by centrifugation at 1600 rpm for 3 min, and resuspend them in a high-sugar medium containing 10% FBSRPMI1640; add 100 μL of HH cell suspension to each well of a 96-well plate to a final concentration of 2×10 4 Cells per well. The compound 1 and compound 2 isolated in Example 1 were dissolved in DMSO and prepared into a storage solution with a final concentration of 10 mM; when used, 10 μL of the storage solution was added to 90 μL of RPMI1640 medium and diluted into a 1 mM use solution; At the same time, 10 μL of DMSO was added to 90 μL of RPMI1640 medium as a negative control solution. Add 0, 1, 2, 2.5, 3, 3.5, 5, 10 μL of the use solution to a 96-well plate to make a concentration gradient of 0, 10, 20, 25, 30, 35, 50, 100 μM; at the same time, negative The...
Embodiment 3
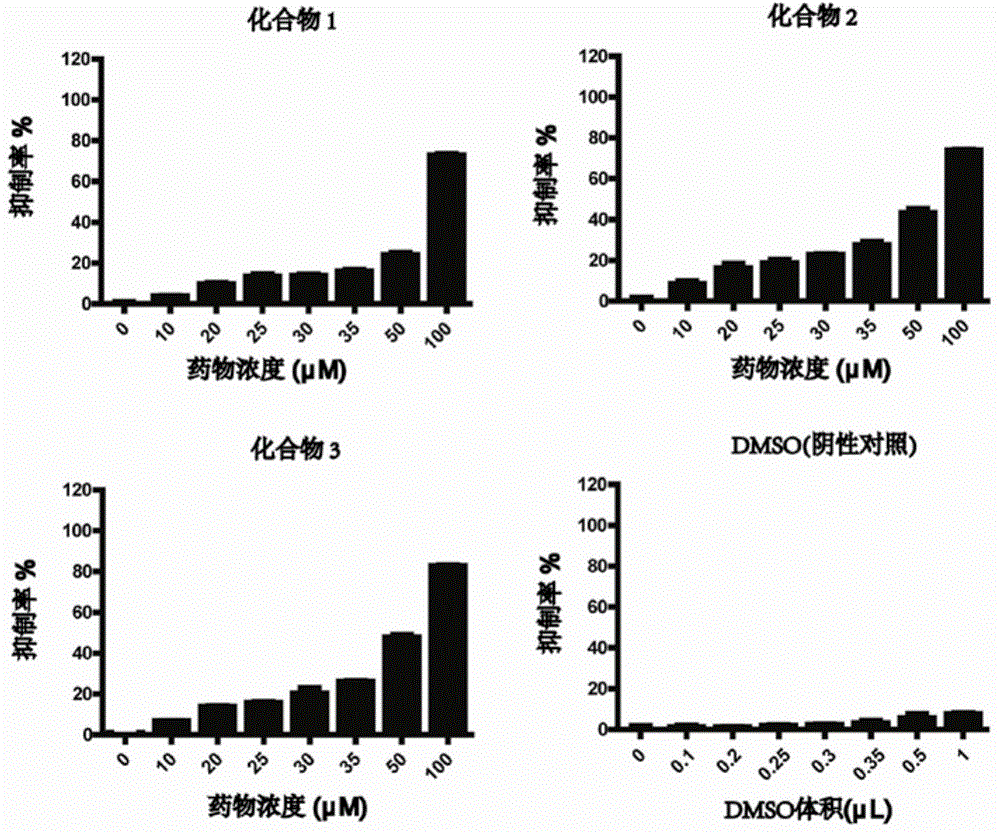
[0035] Example 3: In vitro inhibition of the compound of the present invention on the proliferation of human immortalized epidermal keratinocyte cell line HaCaT cells
[0036] Trypsin-EDTA was used to digest HaCaT cells in the logarithmic growth phase; collected by centrifugation at 1600 rpm for 3 min, and resuspended in DMED high-sugar medium containing 10% FBS; 100 μL of cell suspension was added to each well of a 96-well plate, The final concentration is 5000 cells per well; 37℃, 5% CO 2 Incubate in an incubator for 12 hours; according to the above method, add different volumes of use solution to a 96-well plate to make a concentration gradient of 0, 10, 20, 25, 30, 35, 50, 100 μM; at the same time, add the corresponding negative control group Volume of liquid used in the negative control group. 37°C, 5% CO 2 Incubate in an incubator for 24 hours, add 10 μL of CCK-8 to each well, incubate in the incubator for 4 hours in the dark, measure the absorbance at 450 nm with a micropl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com