Arctigenin ether derivative and preparing method and application thereof
A technology of arctigenin ether and derivatives, which is applied in the field of drug synthesis, can solve the problems of low pass rate, high plasma protein binding rate, low bioavailability, blood-brain barrier, etc., and achieve the effect of reasonable design and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
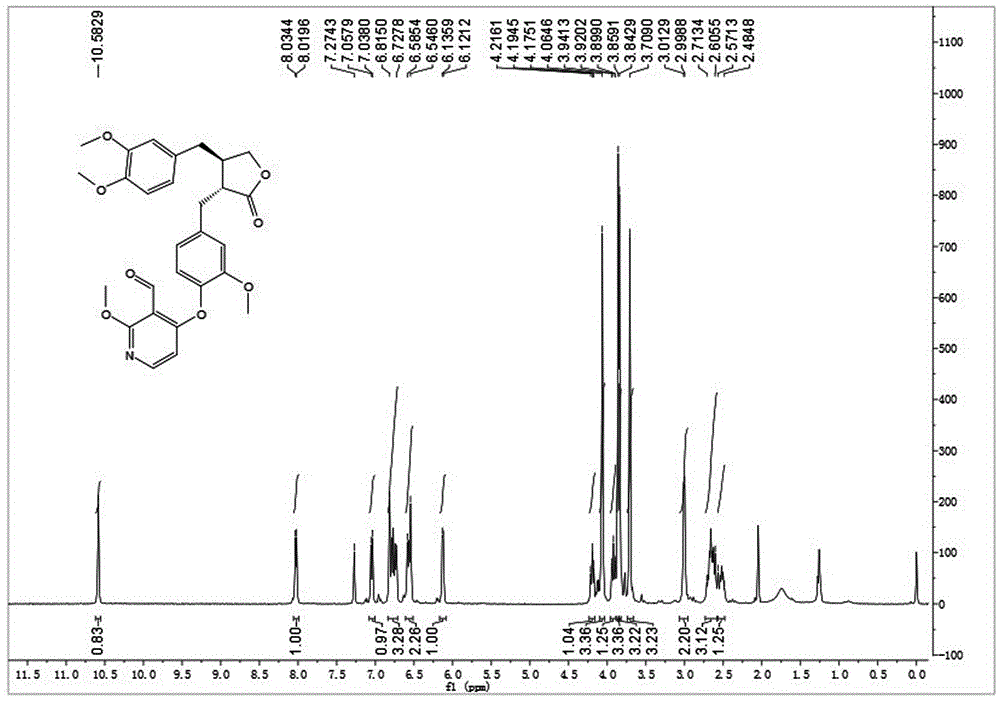
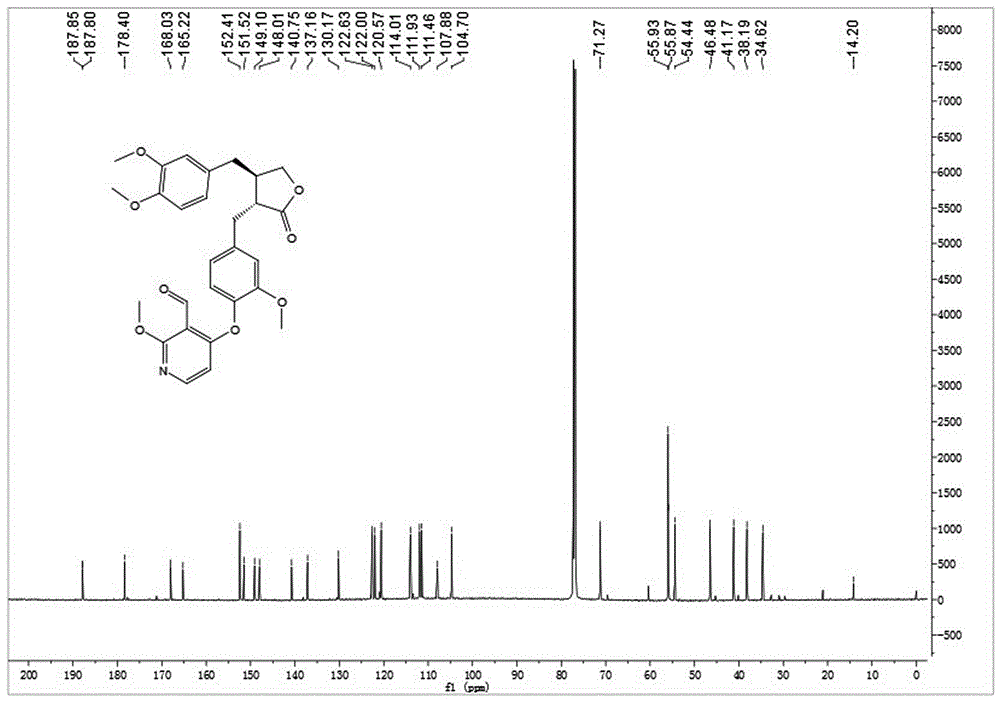
[0035] Embodiment 1: synthetic compound 1-1, please refer to figure 1 and figure 2
[0036] .
[0037] Dissolve arctigenin (50mg, 0.134mmol) in 2ml of dry N,N-dimethylformamide (DMF), add potassium carbonate (37mg, 0.268mmol), stir at room temperature for 10min, then add 4-iodo-2 -Methoxypyridine-3-carbaldehyde (42mg, 0.161mmol), stirred at 120°C for 8h. After cooling to room temperature, 10 mL of ice water was added, the solid precipitated out, filtered with suction, the filter cake was washed with 5% potassium bisulfate solution, dried in vacuo and recrystallized from methanol to obtain 60 mg of white solid with a yield of 88%. [α] D 25 =+3.9(c1.0,CHCl 3 ); 1 H-NMR (400MHz, CDCl 3)δ10.58(s,1H),8.03(d,J=5.9Hz,1H),7.05(d,J=8.0Hz,1H),6.85–6.70(m,3H),6.62–6.52(m,2H ),6.13(d,J=5.9Hz,1H),4.20(t,J=8.2Hz,1H),4.06(s,3H),3.92(t,J=8.5Hz,1H),3.86(s,3H ),3.84(s,3H),3.71(s,3H),3.01(d,J=5.6Hz,2H),2.73–2.58(m,3H),2.56–2.46(m,1H); ESI-MS( m / z):[M+Na] + =530.3(Calcd:507.2).
Embodiment 2
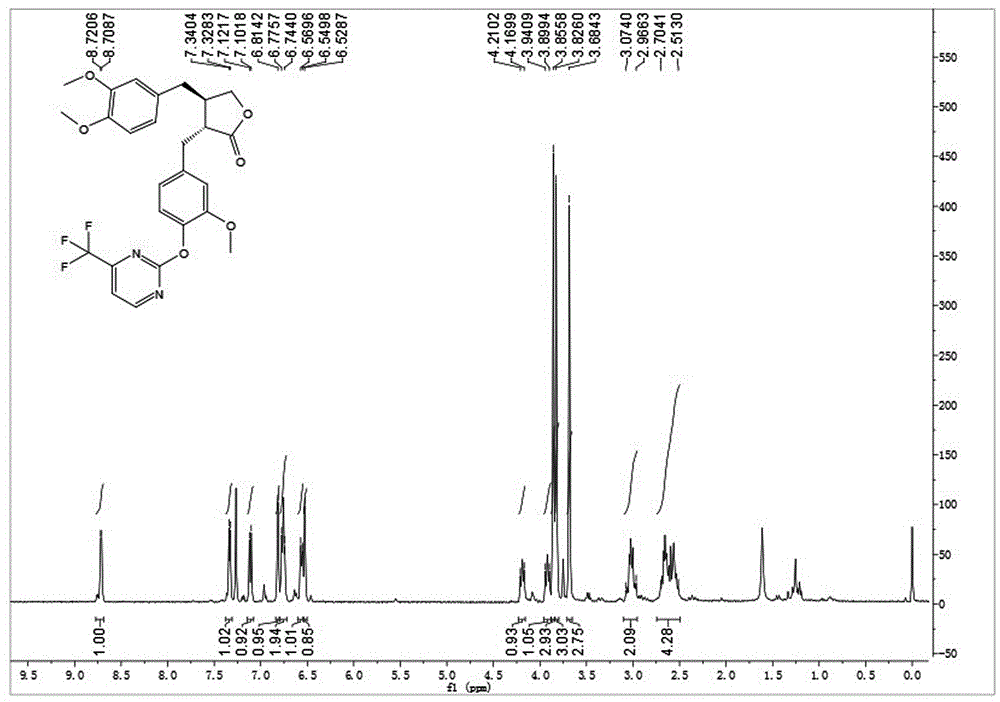
[0038] Embodiment 2: synthetic compound 1-2, please refer to image 3 and Figure 4
[0039] .
[0040] Dissolve arctigenin (50mg, 0.134mmol) in 2ml of dry N,N-dimethylformamide (DMF), add potassium carbonate (37mg, 0.268mmol), stir at room temperature for 10min, then add 2-bromo-3 - Trifluoromethylpyrimidine (37mg, 0.161mmol), stirred at 120°C for 8h. After cooling to room temperature, 10 mL of ice water was added, and the solid precipitated out. Suction filtration, the filter cake was washed with 5% potassium bisulfate solution, dried in vacuo, and methanol was recrystallized to obtain 64 mg of a white solid, with a yield of 92%. The white solid, [α] D 20 =-1.5(c1.0, CHCl 3 ); 1 HNMR (400MHz, CDCl 3 )δ8.71(d,J=4.8Hz,1H),7.33(d,J=4.8Hz,1H),7.11(d,J=8.0Hz,1H),6.81(s,1H),6.78–6.74( m,2H),6.59–6.54(m,1H),6.53(s,1H),4.23–4.15(m,1H),3.95–3.88(m,1H),3.86(s,3H),3.83(s, 3H), 3.68(s,3H), 3.09–2.96(m,2H), 2.72–2.50(m,4H); ESI-MS(m / z):[M+Na] + =540.8(Calcd:518.1).
Embodiment 3
[0041] Embodiment 3: synthetic compound 1-3, please refer to Figure 5 and Figure 6
[0042] .
[0043] Arctigenin (50mg, 0.134mmol) was dissolved in 2ml of acetone, potassium carbonate (37mg, 0.268mmol) and 3-nitrobromobenzene (33mg, 0.161mmol) were added successively, and refluxed for 4h. Remove the solvent under reduced pressure, add 20 mL of water, filter with suction, wash the filter cake several times with 5% potassium bisulfate solution, and recrystallize methanol after vacuum drying to obtain 42 mg of light yellow solid with a yield of 63%. [α] D 20 =-2.1(c1.0,CHCl 3 ); 1 HNMR (400MHz, CDCl 3 )δ7.85(d,J=7.0Hz,1H),7.62(s,1H),7.41(t,J=8.2Hz,1H),7.21(dd,J=8.2,1.4Hz,1H),6.97( d,J=8.0Hz,1H),6.82(s,1H),6.76(d,J=8.1Hz,1H),6.71(d,J=8.0Hz,1H),6.57(d,J=8.0Hz, 1H),6.53(s,1H),4.19(dd,J=8.7,7.6Hz,1H),3.91(t,J=8.4Hz,1H),3.83(s,3H),3.81(s,3H), 3.72(s,3H),2.99(d,J=5.6Hz,2H),2.71–2.47(m,4H);ESI-MS(m / z):[M+Na] + =516.8(Calcd:493.2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com