Method for preparing cefditoren pivoxil cephalosporins
A technology for cefditoren pivoxil and cephalosporin, which is applied in the field of preparation of cefditoren pivoxil, can solve the problems of many by-products, difficult product purification, low reaction yield, etc. Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
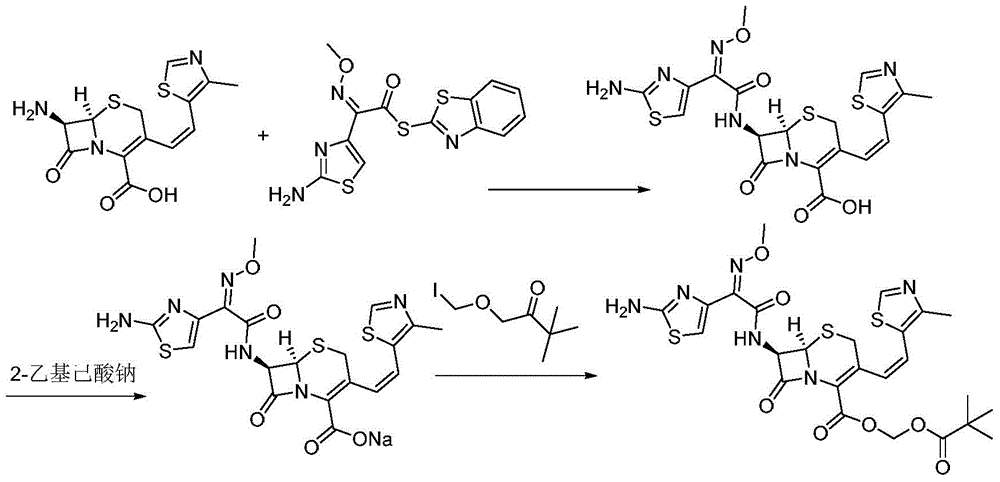
[0030] A preparation method of cefditoren pivoxil, the preparation method comprising the following steps:
[0031] 1) In the presence of TMEDA and sodium phosphate, add 7-ATCA (32.3g, 100mmol) and MAEM (38.8g, 110mmol) to the reaction kettle and contact for 2 hours in 300mL THF to obtain a mixture containing ceftoren acid. Contact reaction The temperature is 12°C; wherein the molar ratio of 7-ATCA to TMEDA and sodium phosphate is 1:1.6:0.35;
[0032] 2) Keep the temperature, add TEA (15.2g, 150mmol) to the mixture containing cefditoren acid obtained in step 1), then add iodomethyl pivalate (31.5g, 130mmol) to the above mixture and stir to react , Keep the reaction for 30 minutes, pour into water after the reaction, the amount of water is twice the volume of the amount of THF in step 1), add saturated ammonium chloride solution to adjust the pH to 5.1, filter, and filter the filter cake Cefditoren pivoxil was obtained by recrystallization from methanol, and 55.6 g of white solid wa...
Embodiment 2
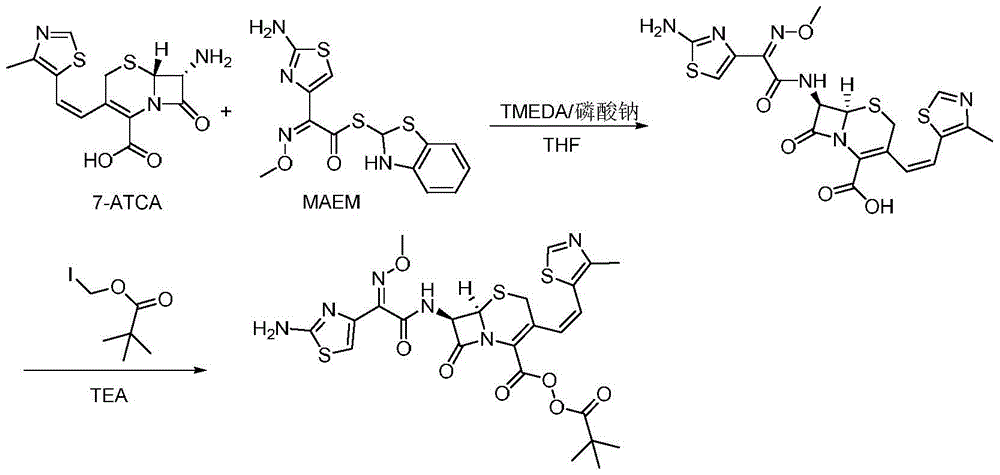
[0034] A preparation method of cefditoren pivoxil, the preparation method comprising the following steps:
[0035] 1) In the presence of TMEDA and sodium phosphate, 7-ATCA (32.3g, 100mmol) and MAEM (42.3g, 120mmol) were added to the reaction kettle and contacted in 300mLTHF for 2.5 hours to obtain a mixture containing ceftoren acid. Contact reaction The temperature is 15°C; among them, the molar ratio of 7-ATCA to TMEDA and sodium phosphate is 1:2:0.2;
[0036] 2) Keep the temperature, add TEA (25.3g, 250mmol) to the mixture containing cefditoren acid obtained in step 1), and then add iodomethyl pivalate (36.3g, 150mmol) to the above mixture and stir to react , Keep the reaction for 25 minutes, pour into water after the reaction, the amount of water is 3 times the volume of the amount of THF in step 1), add saturated ammonium chloride solution to adjust the pH to 5, filter, and filter the filter cake Cefditoren pivoxil was obtained by recrystallization from methanol, and 54.5 g of...
Embodiment 3
[0038] A preparation method of cefditoren pivoxil, the preparation method comprising the following steps:
[0039] 1) In the presence of TMEDA and sodium phosphate, add 7-ATCA (32.3g, 100mmol) and MAEM (37g, 105mmol) to the reaction kettle and contact for 2 hours in 300mLTHF to obtain a mixture containing ceftoren acid. The temperature is 10°C; wherein the molar ratio of 7-ATCA to TMEDA and sodium phosphate is 1:1.5:0.5;
[0040] 2) Keep the temperature, add TEA (11.1g, 110mmol) to the mixture containing ceftoren acid obtained in step 1), then add iodomethyl pivalate (31.5g, 130mmol) to the above mixture and stir to react , Keep the reaction for 30 minutes, pour into water after the reaction, the amount of water is 2.5 times the volume of the amount of THF in step 1), add saturated ammonium chloride solution to adjust the pH to 5.2, filter, and filter the filter cake Cefditoren pivoxil was obtained by recrystallization from methanol, and 53.6 g of white solid was obtained by vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com