Continuous release composition made of hyaluronic acid and its therapeutic use
A technology of hyaluronic acid and hyaluronic acid salt, applied in the field of preventing skin aging and/or repairing dermal tissue, which can solve problems such as uncontrollable size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Preparation of polymer particles according to the invention:
[0075] Step 1: "Primary" Emulsification
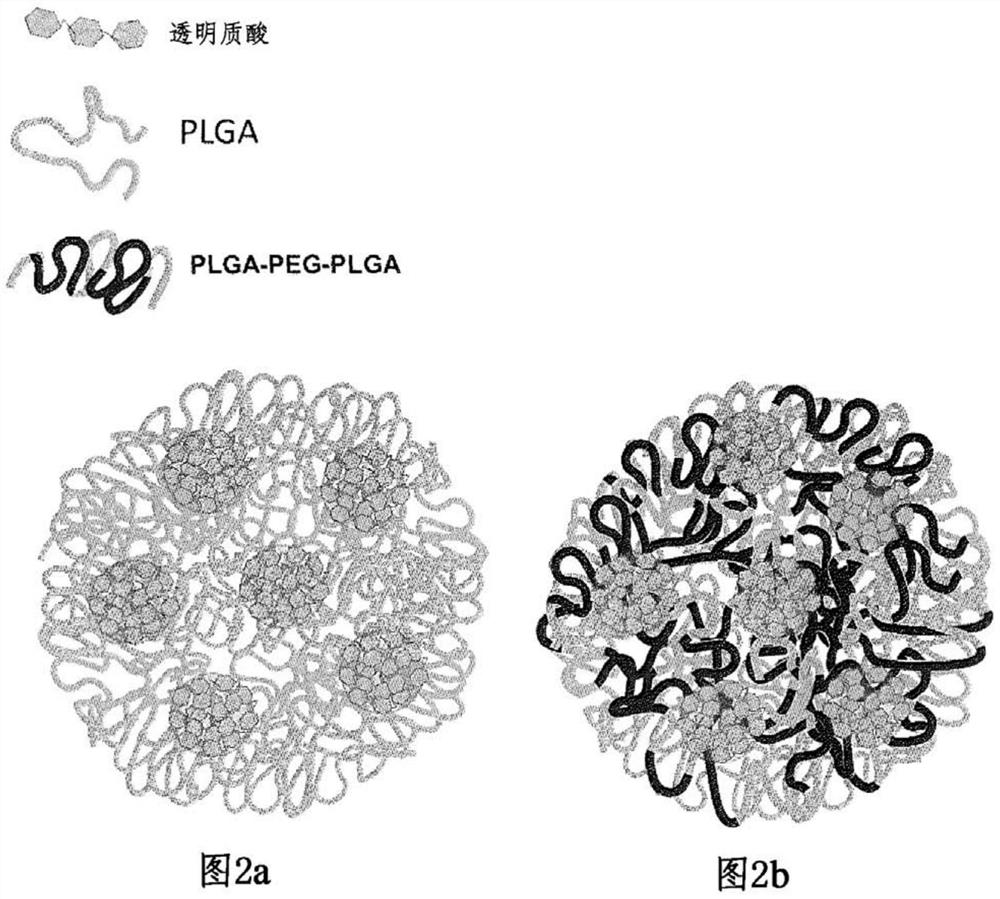
[0076] An aqueous hyaluronic acid solution was prepared by dissolving 50 mg of hyaluronic acid in 5 ml of a 4 wt % polyvinyl alcohol (PVA) solution. Separately, an organic solution of the polymer was prepared by dissolving 900 mg of PLGA-PEG-PLGA triblock polymer in 12 ml of dichloromethane / acetone (3 / 1 v / v) mixture. use The two solutions were emulsified at room temperature for two minutes with an IKA T25 basic stirrer at 16,000 rpm while magnetically stirring at a rate of 500 rpm with a magnetic bar. A second stirring cycle was performed in the same manner in an ice bath.
[0077] Step 2: "Secondary" Emulsification
[0078] The stable emulsion obtained from step 1 was introduced into a glass syringe and injected into a formulation reactor containing 450 ml of 4 wt% polyvinyl alcohol (PVA) under magnetic stirring at a rate of 750 rpm.
[0079] Step 3: Addition ...
Embodiment 2
[0114] Preparation of polymer particles according to the invention:
[0115] The polymer particles according to the invention were prepared in a similar manner to Example 1. The only change relative to the method of Example 1 is that a Silverson stirrer at a rate of 2800 rpm was used instead of the magnetic stirring at 750 rpm in Example 1 in the "secondary" emulsification preparation in the second step.
[0116] Analysis of the inventive polymer particles according to Example 2:
[0117] Ten batches of polymer particles according to the invention were analyzed. The results obtained are as follows:
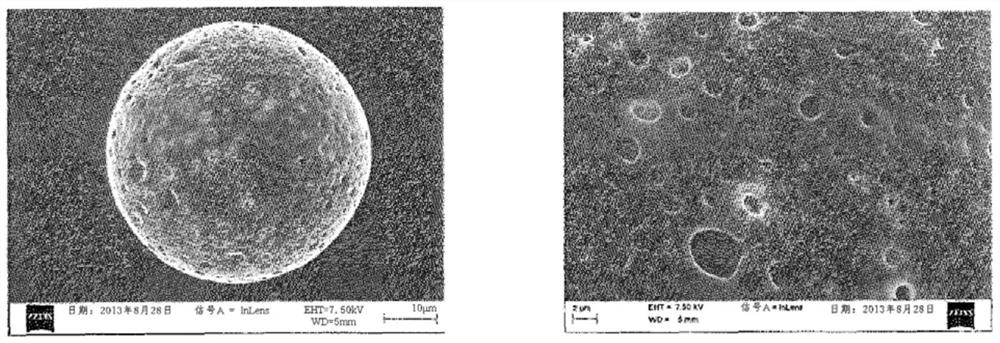
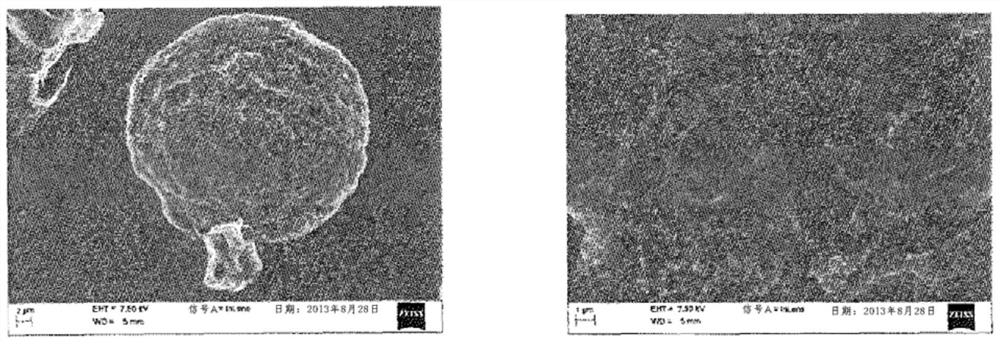
[0118] - according to use 3 Particle size analysis of a Coulter counter (Beckman Coulter), the size of the particles obtained from polydispersity: 34.98 μm ± 7.02 μm (before irradiation) and 34.12 μm ± 7.23 μm (after irradiation),
[0119] - content of hyaluronic acid: 16 μg of hyaluronic acid loaded per mg of freeze-dried polymer particles, and
[0120] - The binding yield ...
Embodiment 3
[0128] Preparation of polymer particles according to the invention:
[0129] A method similar to that of Example 2 was used for the preparation of the polymer particles according to the invention. The only change relative to the method of Example 2 is the addition of a pharmaceutically acceptable excipient which is also anti-inflammatory (radical scavenger), i.e. 1 ml of sorbitol in the form of an ultrapure aqueous solution of 10 wt% sorbitol Substitute the ultrapure water used in Example 2.
[0130] Analysis of the inventive polymer particles according to Example 3:
[0131] Six batches of polymer particles according to the invention were analyzed. The results obtained are as follows:
[0132] - according to use 3 Particle size analysis by Coulter counter (Beckman Coulter), the size of the particles obtained from the polydispersity: 31.15 μm ± 7.80 μm, and
[0133] - The content of hyaluronic acid is: 13.94 μg of hyaluronic acid is loaded per mg of freeze-dried polymer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com