Preparation method of choline fenofibrate acid sustained release capsule
A technology of fenofibric acid choline and sustained-release capsules, which is applied in the direction of non-active ingredient medical preparations, capsule delivery, microcapsules, etc., and can solve problems such as poor sustained-release effect and lack of fenofibric acid choline , to achieve the effect of fast and complete release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation method of fenofibric acid choline sustained-release capsules, the steps are as follows:
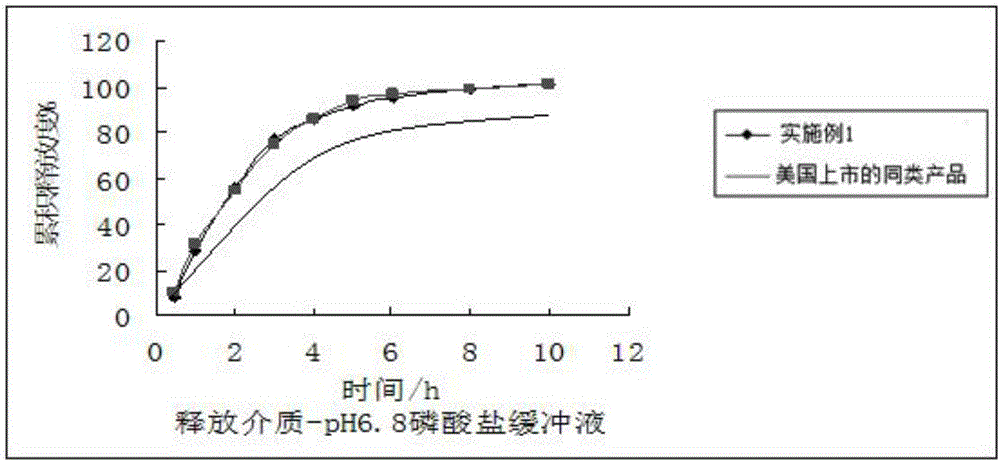
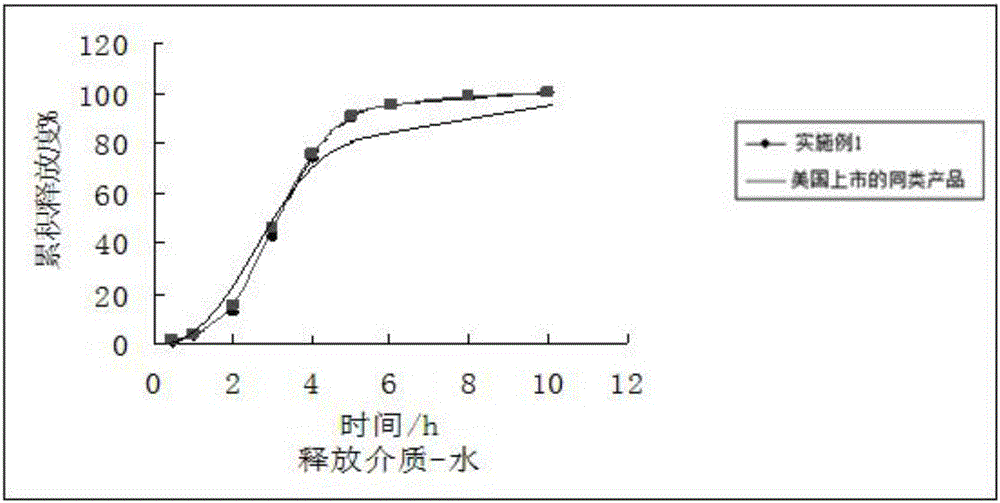
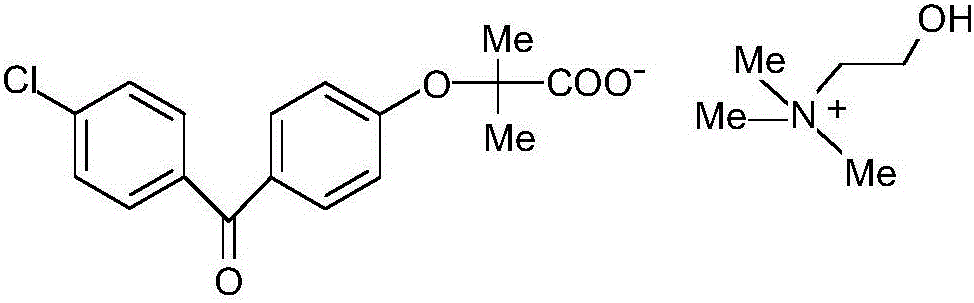
[0018] 1) Preparation of drug-loaded pellet core: Dissolve fenofibric acid choline with 3 times the weight of ethanol, add microcrystalline cellulose blank pellet core (purchased from JRS Pharmaceutical Excipient Company, Germany) into the fluidized bed, and add fenofibrate Spray the choline fenofibric acid ethanol solution onto the blank pellet core, control the atomization pressure at 2.5-3bar when applying the drug, the peristaltic pump speed at 100-120rpm, and the material temperature at 35-42°C. When the quality of fenofibric acid choline reaches When the mass of the blank ball core is 50%, stop applying the medicine and dry at 65°C for 2 hours;
[0019] 2) film coating: spray film coating agent on the drug-loaded ball core in fluidized bed, the raw material weight proportion of described film coating agent is ethyl cellulose 9%, dibutyl sebacate 1% , 45% etha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com