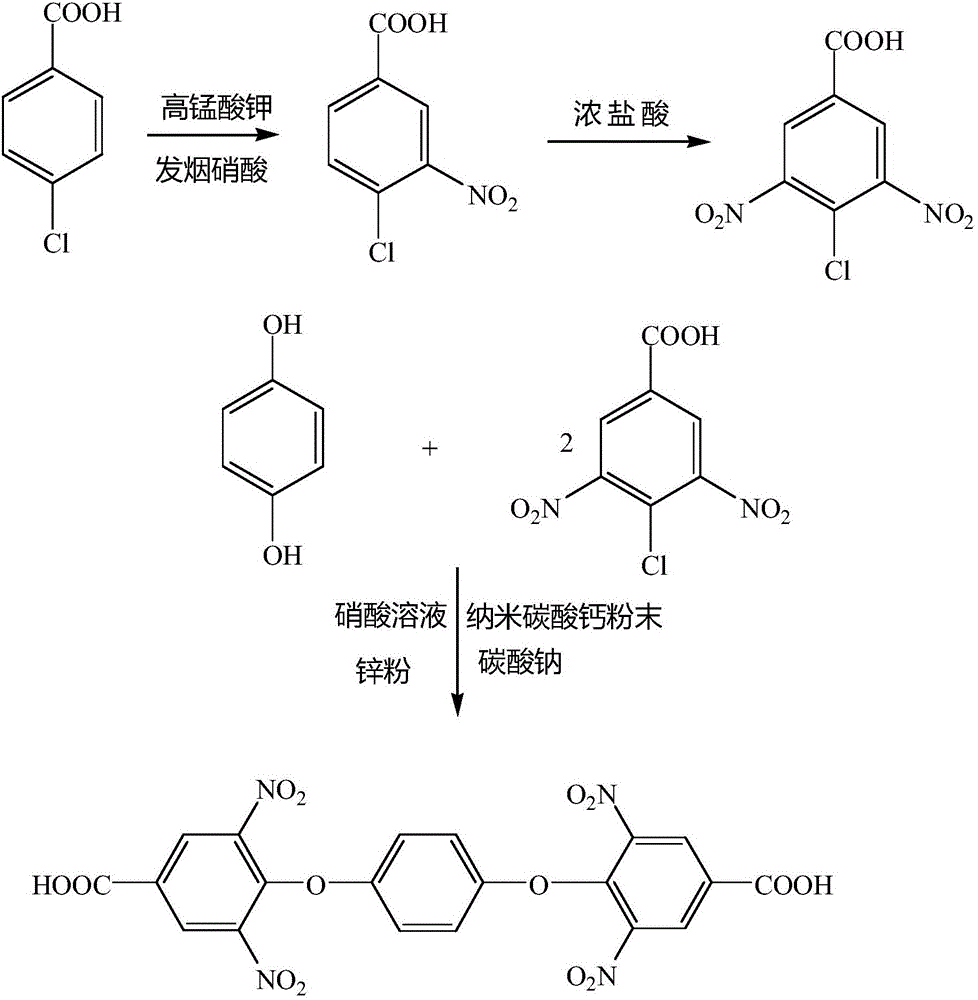
Synthesis method for 1,4-bis(2,6-binitro-4-formic acid phenoxy)benzene
A synthetic method and dinitro technology, which is applied in the preparation of nitro compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as infusibility, insolubility, and difficult molding processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0014] In a 1000mL three-necked flask equipped with a stirring device, add 400mL of distilled water, 35g of potassium permanganate and 25g of p-chlorotoluene, put the flask in an oil bath, raise the temperature to 120°C, and keep reflux at this temperature 1h, after stirring, add 12g of potassium permanganate to the bottle, and continue to reflux for 50min. After the reaction is over, evaporate excess p-chlorotoluene at 75°C, and add 10g of fuming nitric acid to it after evaporation , and control the temperature below 60°C, stir for 20 minutes after the dropwise addition is completed, cool after stirring, perform suction filtration, and dry the obtained solid in an oven at 45°C to obtain 3,5-dinitro-4-chlorobenzoic acid ; In a 500mL three-necked flask equipped with a thermometer and a stirring device, add 10g hydroquinone and 5g sodium carbonate, and 12g3,5-dinitro-4-chlorobenzoic acid and 50mL toluene, put it into In an oil bath, raise the temperature to 120°C and stir for 30...
example 2
[0016] In a 1000mL three-neck flask equipped with a stirring device, add 450mL of distilled water, 40g of potassium permanganate and 27g of p-chlorotoluene, put the flask in an oil bath, raise the temperature to 125°C, and keep reflux at this temperature 2h, after stirring, add 13g of potassium permanganate to the bottle, and continue to reflux for 60min. After the reaction is over, evaporate excess p-chlorotoluene at 80°C, and add 11g of fuming nitric acid to it after evaporation , and control the temperature below 60°C, stir for 25 minutes after the dropwise addition is completed, cool after stirring, perform suction filtration, and dry the obtained solid in an oven at 50°C to obtain 3,5-dinitro-4-chlorobenzoic acid ; In a 500mL three-necked flask equipped with a thermometer and a stirring device, add 13g hydroquinone and 6g sodium carbonate, and 13g3,5-dinitro-4-chlorobenzoic acid and 55mL toluene, put it into In the oil bath, raise the temperature to 125°C and stir for 35 ...
example 3
[0018]In a 1000mL three-necked flask equipped with a stirring device, add 500mL of distilled water, 45g of potassium permanganate and 30g of p-chlorotoluene, put the flask in an oil bath, raise the temperature to 130°C, and keep reflux at this temperature 3h, after the stirring is over, add 15g of potassium permanganate to the bottle, and continue to reflux for 70min. After the reaction is over, evaporate the excess p-chlorotoluene at 85°C, and add 12g of fuming nitric acid to it after evaporation , and control the temperature below 60°C, stir for 30 minutes after the dropwise addition is completed, cool after stirring, perform suction filtration, and dry the obtained solid in an oven at 55°C to obtain 3,5-dinitro-4-chlorobenzoic acid ; In a 500mL three-neck flask equipped with a thermometer and a stirring device, add 15g hydroquinone and 7g sodium carbonate, and 15g3,5-dinitro-4-chlorobenzoic acid and 60mL toluene, put it into In an oil bath, raise the temperature to 130°C an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com