Environmentally friendly preparation technology of 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane
A technology of hydroxyphenyl and hexafluoropropane, which is applied in the field of 2,2-dihexafluoropropane preparation process, can solve problems affecting the quality of reaction products, corrosion, damage to equipment, etc., and reduce the generation of toxic, harmful and high-risk by-products , the effect of eliminating pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
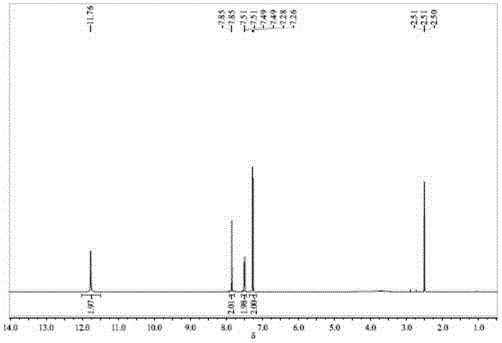
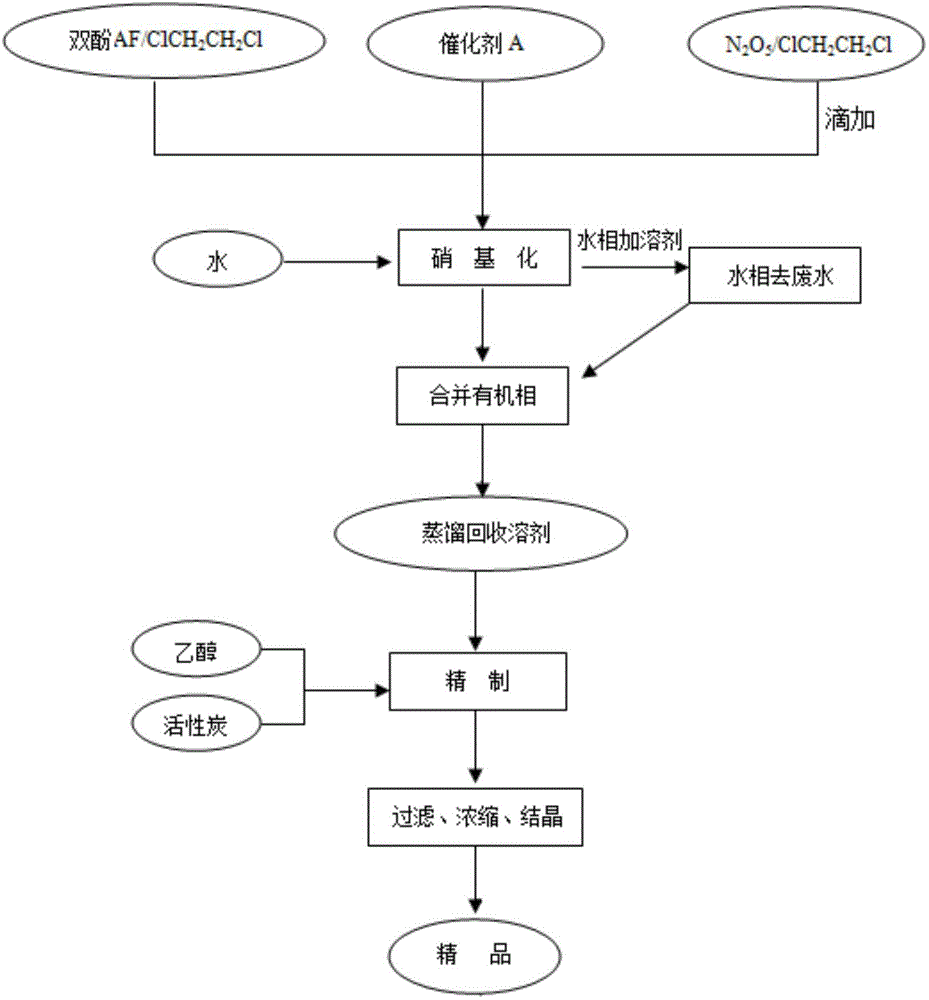
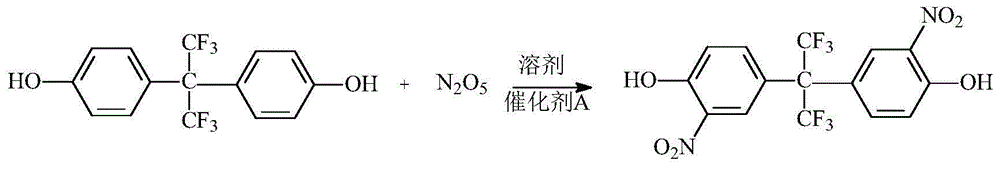
[0024] In a 500 ml three-necked flask equipped with an electric stirrer, add 67 grams of bisphenol AF and 335 grams of 1,2-dichloroethane, dissolve under stirring, add 1.5 grams of catalyst A, cool in an ice bath, and at 20 ° C Add 100ml of 12mol / L N dropwise 2 o 5 / 1,2-dichloroethane solution, after the dropwise addition is completed, the temperature is raised to 30-45°C, and the reaction is continued for 4 hours. After the reaction, filter, pour the filtrate into a separatory funnel, add about 60 ml of 5% sodium carbonate solution and deionized water to wash to neutrality, separate the organic layer, and continue to use about 50 grams of 1,2 -Dichloroethane was extracted and separated, the organic phases were combined, dried by adding 30 grams of anhydrous sodium sulfate, filtered, and the filtrate was distilled under negative pressure (about 400-500kPa vacuum) to recover 1,2-dichloroethane, and the residual solid Add 300 milliliters of absolute ethanol and 1 gram of activa...
Embodiment 2
[0027] In a 500 ml three-neck flask equipped with an electric stirrer, add 67 grams of bisphenol AF and 268 grams of 1,2-dichloroethane, dissolve under stirring, add 1.5 grams of catalyst A, cool in an ice bath, and at 0 ° C Add 120ml of 12mol / L N dropwise 2 o 5 / 1,2-dichloroethane solution, after the dropwise addition is completed, the temperature is raised to 20-30°C, and the reaction is continued for 5 hours. After the reaction, filter, pour the filtrate into a separatory funnel, add about 60 ml of 5% sodium carbonate solution and deionized water to wash to neutrality, separate the organic layer, and continue to use about 50 grams of 1,2 -Dichloroethane was extracted and separated, the organic phases were combined, dried by adding 30 grams of anhydrous sodium sulfate, filtered, and the filtrate was distilled under negative pressure (about 400-500kPa vacuum) to recover 1,2-dichloroethane, and the residual solid Add 300 milliliters of absolute ethanol and 1 gram of activate...
Embodiment 3
[0030] In a 500 ml three-necked flask equipped with an electric stirrer, add 67 grams of bisphenol AF and 268 grams of 1,2-dichloroethane, dissolve under stirring, add 1.5 grams of catalyst A, cool in an ice bath, and at 10 ° C Add 120ml of 12mol / L N dropwise 2 o 5 / 1,2-dichloroethane solution, after the dropwise addition is completed, the temperature is raised to 15-20°C, and the reaction is continued for 6 hours. After the reaction, filter, pour the filtrate into a separatory funnel, add about 60 ml of 5% sodium carbonate solution and deionized water to wash to neutrality, separate the organic layer, and continue to use about 50 grams of 1,2 -Dichloroethane was extracted and separated, the organic phases were combined, dried by adding 30 grams of anhydrous sodium sulfate, filtered, and the filtrate was distilled under negative pressure (about 400-500kPa vacuum) to recover 1,2-dichloroethane, and the residual solid Add 300 milliliters of absolute ethanol and 1 gram of activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com