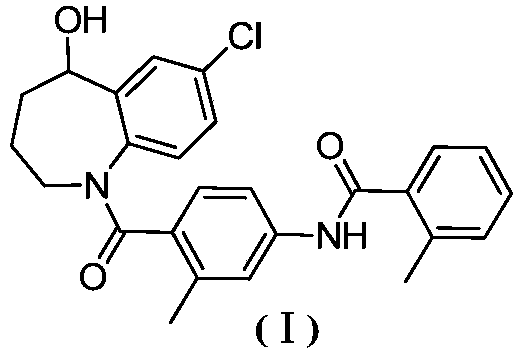
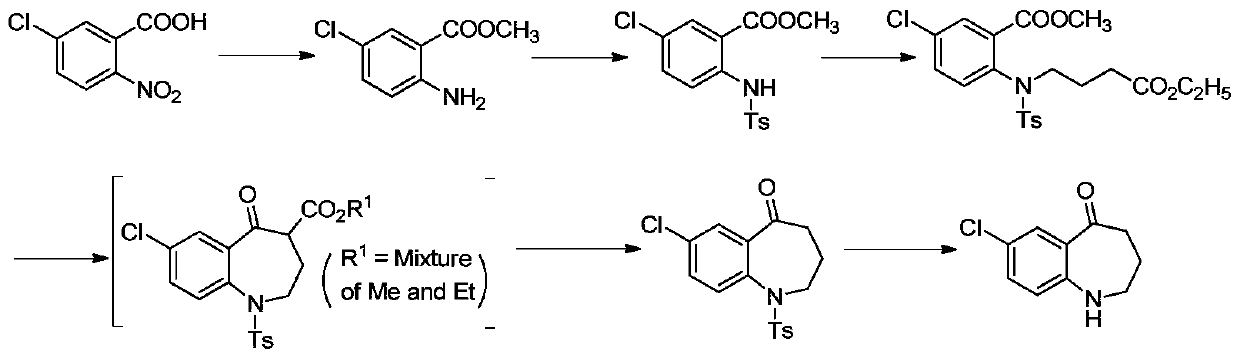
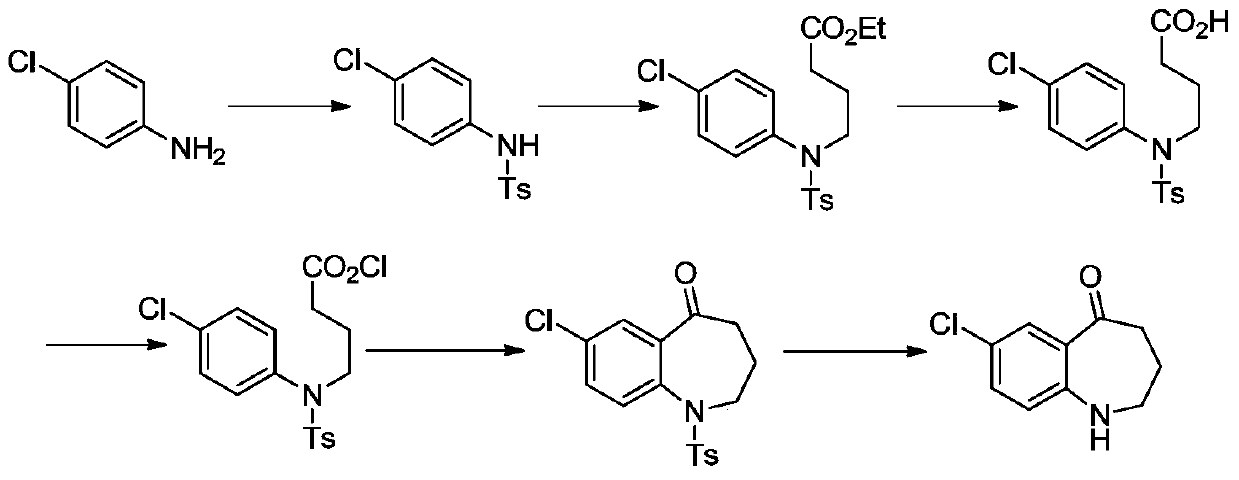
A kind of preparation method of high-efficiency and low-toxicity vasopressin antagonist drug
A halogen and compound technology, which is applied in the field of preparation of tolvaptan, a selective non-peptidin arginine vasopressin V2 receptor antagonist, can solve problems such as difficulties in the preparation of analogues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0136] Specifically, the preparation method of the compound of the formula (VIII) comprises the steps:
[0137] (4) in an inert solvent, react with the compound of formula (VII) to obtain the compound of formula (VIII);
[0138]
[0139] Preferably, described step (4) comprises the following sub-steps:
[0140] (4.1) in an inert solvent, the compound of formula (VII) is reacted with a chlorinating reagent to obtain a compound of formula B;
[0141]
[0142] (4.2) in an inert solvent, in the presence of a catalyst, perform cyclization with a compound of formula B to obtain a compound of formula (VIII);
[0143]
[0144] The reaction conditions are not particularly limited. In another preferred example, in the step (4.1), the chlorinating reagent is selected from the following group: chlorine, NCS, thionyl chloride, trichloride phosphorus oxychloride, phosphorus oxychloride, phosphorus pentachloride, trichloroisocyanuric acid, or a combination thereof.
[0145] In an...
Embodiment 1
[0207] (1) Synthesis of compound (IV)
[0208] p-Chloroaniline (20.0 g, 156.78 mmol) and ethyl 4-bromobutyrate (III) (30.58 g, 156.78 mmol) were dissolved in acetonitrile (500 mL), sodium carbonate (32.23 g, 313.55 mmol) was added, followed by stirring It was heated to 80° C., reacted for 4 h, detected by TLC, and the reaction was completed. Cool to room temperature, pour the reaction solution into water (300 mL), take the organic phase, extract the aqueous phase twice with ethyl acetate (200 mL x 2), combine the organic phases, and wash twice with water (200 mL x 2), anhydrous It was dried with sodium sulfate, and the solvent was spun off to obtain 32.21 g of compound (IV) with a yield of 85.0%.
[0209] (2) Synthesis of compound (VI)
[0210] Compound (IV) (32.0 g, 132.39 mmol) was dissolved in dichloromethane (600 mL), triethylamine (36.9 mL, 264.78 mmol) was added under stirring, and 2-methyl-4-nitrogen was slowly added at room temperature benzoyl chloride (V) (31.71 g,...
Embodiment 2
[0224] (1) Synthesis of Compound (Ⅳ)
[0225] Dissolve p-chloroaniline (20.0g, 156.78mmol) and ethyl 4-bromobutyrate (Ⅲ) (30.58g, 156.78mmol) in acetonitrile (500mL), add potassium carbonate (43.34g, 313.55mmol), and stir Heated to 80°C, reacted for 4h, and detected by TLC, the reaction was complete. Cool to room temperature, pour the reaction solution into water (300mL), take the organic phase, extract the aqueous phase with ethyl acetate twice (200mL x 2), combine the organic phases, and wash twice with water (200mL x 2), anhydrous After drying over sodium sulfate, the solvent was spun off to obtain 32.97 g of compound (IV), with a yield of 87.0%.
[0226] (2) Synthesis of compound (Ⅵ)
[0227] Compound (Ⅳ) (32.0g, 132.39mmol) was dissolved in dichloromethane (600mL), diisopropylethylamine (34.22g, 264.78mmol) was added under stirring, and 2-methyl- 4-Nitrobenzoyl chloride (Ⅴ) (31.71g, 158.87mmol), reacted for 3h, and TLC detected that the reaction was complete. The reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com