Production method for improving pyrithione zinc whiteness
A technology of zinc pyrithione and production method, applied in directions such as organic chemistry, can solve the problems such as not introducing a method for improving the whiteness of zinc pyrithione, and achieve the effects of easy operation, stable quality and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
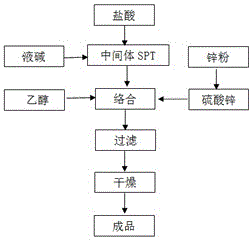
[0021] Example 1: In the first step of zinc sulfate purification, 4 kg of zinc powder is added to 5000 kg of 30% zinc sulfate solution at a temperature of 60 ° C, and the heavy metal ions of iron, nickel, copper, chromium and cobalt are removed by filtration with a 0.45 μm filter membrane , so that the heavy metal content is less than 1PPM; through sampling and detection, use atomic absorption spectroscopy equipment to detect whether the heavy metal content is 1PPM. In the second step acid stripping reaction, adding concentration to 5000kg of sodium pyrithione is 30% hydrochloric acid, the pH value is adjusted to 3, and the intermediate SPT solution after the reaction is filtered and washed to remove impurities; in the third step In the salt-forming reaction, adding a concentration of 30% sodium hydroxide solution to the intermediate SPT solution dissolves, and generates sodium pyrithione solution again, so that the concentration of sodium pyrithione is 15%, and the pH value is...
Embodiment 2
[0023] Example 2: In the first step of zinc sulfate purification, add 3kg of zinc powder to 3000kg of zinc sulfate solution with a concentration of 15%, at a temperature of 50°C, and remove iron, nickel, copper, chromium, and cobalt heavy metal ions by filtering through a 0.45 μm filter membrane , so that the heavy metal content is less than 1PPM; in the second step acid stripping reaction, adding concentration to 3000kg sodium pyrithione is 15% hydrochloric acid, the pH value is adjusted to 4, and the intermediate SPT solution after the reaction is filtered and washed, Remove impurities; in the third step of salt-forming reaction, add a sodium hydroxide solution with a concentration of 15% to the intermediate SPT solution to dissolve, and generate sodium pyrithione solution again, so that the concentration of sodium pyrithione is 15%, and the pH value At 9; the sodium pyrithione solution obtained in the third step is adjusted to a pH value of 6 with hydrochloric acid of 15% co...
Embodiment 3
[0025] Example 3: In the first step of zinc sulfate purification, 5 kg of zinc powder is added to 10000 kg of 32% zinc sulfate solution at a temperature of 80 ° C, and the heavy metal ions of iron, nickel, copper, chromium and cobalt are removed by filtration with a filter membrane of 0.45 μm , so that the heavy metal content is less than 1PPM; in the second step acid stripping reaction, adding concentration to 10000kg of sodium pyrithione is 32% hydrochloric acid, the pH value is adjusted to 2, and the intermediate SPT solution after the reaction is filtered and washed. Remove impurities; in the third step of salt-forming reaction, add 50% sodium hydroxide solution to the intermediate SPT solution to dissolve, and generate sodium pyrithione solution again, so that the concentration of sodium pyrithione is 20%, and the pH value At 11; the sodium pyrithione solution obtained in the third step is adjusted to a pH value of 8 with the hydrochloric acid of 32% concentration or the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com