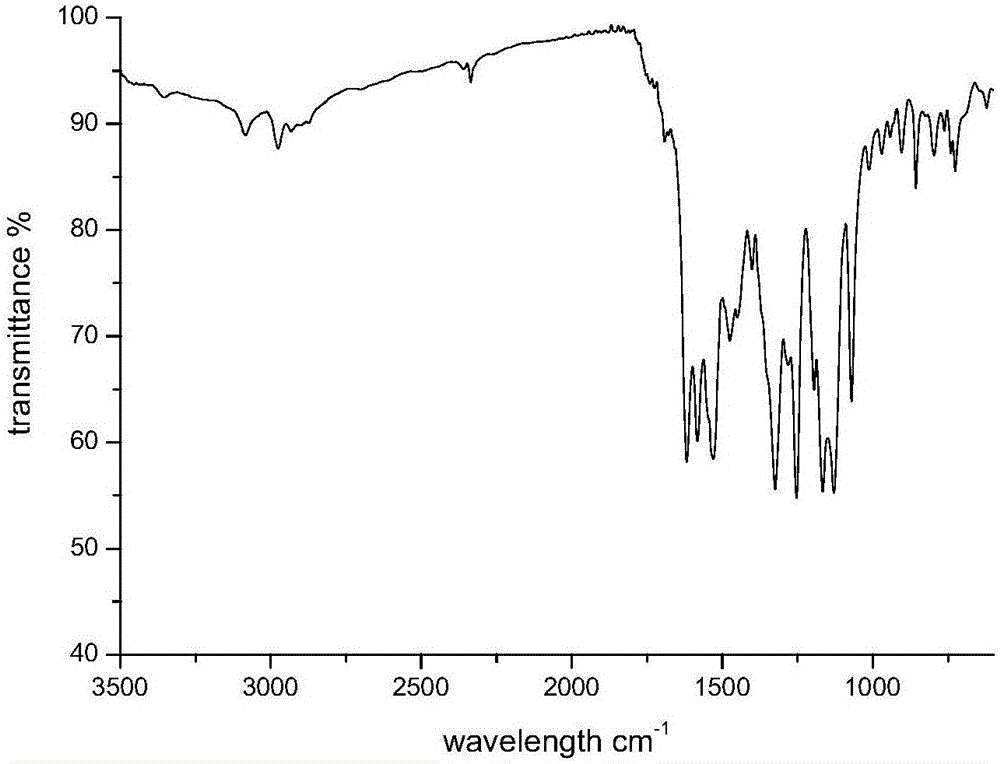
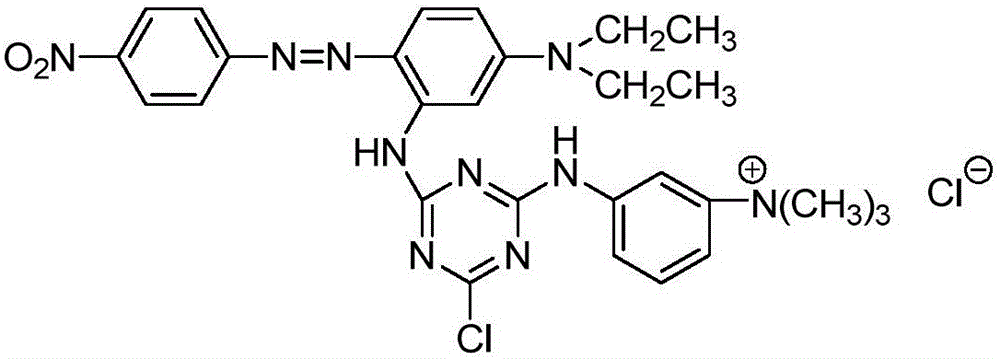
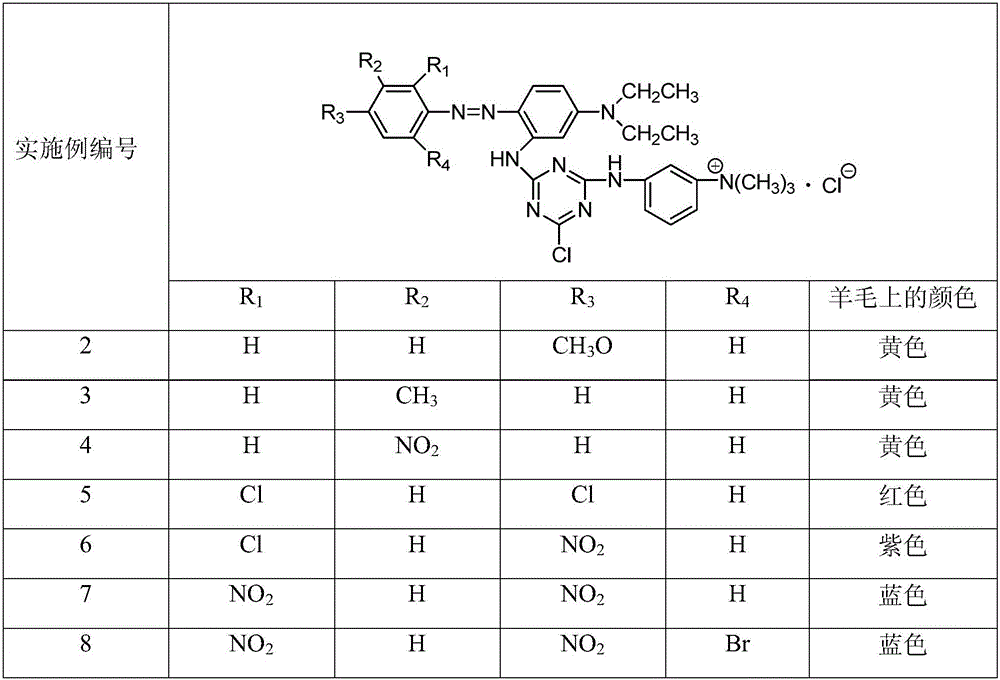
Cationic reactive dye using N,N-diethyl-m-amino aniline as coupling component and preparation method and application thereof
A technology for coupling components and aminoaniline, applied in the direction of reactive dyes, azo dyes, dyeing methods, etc., can solve the problems of increasing production costs, affecting color light and fastness, and high energy consumption, so as to eliminate staining defects, Good color fastness and water saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] a. Dissolve 15g of m-aminoacetanilide in 100ml of deionized water, slowly add 40ml of dimethyl sulfate to the aqueous solution of m-aminoacetanilide dropwise at 65°C and pH=7, react for 12 hours, cool to room temperature, add 10ml of concentrated sulfuric acid was deacetylated at 90°C for 1 hour to obtain the intermediate 3-amino-N,N,N-trimethylbenzyl ammonium sulfate solution, which was directly used in the next step without filtration;
[0026] b. Dissolve 2.77g of cyanuric chloride in 70ml of acetone, and add 10g of crushed ice to make a slurry, and use 2mol / L NaOH solution to dissolve the intermediate 3-amino-N,N,N-trimethylbenzene prepared in step a After the pH value of the ammonium sulfate solution was adjusted to neutral, 25ml of it was slowly added dropwise to the acetone reaction solution of cyanuric chloride, and crushed ice was added to maintain the reaction temperature at 0-5°C. 3 The pH value of the solution was controlled at 4, and a condensation intermed...
Embodiment 2
[0033]a. Dissolve 15g of m-aminoacetanilide in 100ml of deionized water, slowly add 40ml of dimethyl sulfate to the aqueous solution of m-aminoacetanilide dropwise at 65°C and pH=7, react for 12 hours, cool to room temperature, add 10ml of concentrated sulfuric acid was deacetylated at 90°C for 1 hour to obtain the intermediate 3-amino-N,N,N-trimethylbenzyl ammonium sulfate solution, which was directly used in the next step without filtration;
[0034] b. Dissolve 2.77g of cyanuric chloride in 70ml of acetone, and add 10g of crushed ice to make a slurry, and use 2mol / L NaOH solution to dissolve the intermediate 3-amino-N,N,N-trimethylbenzene prepared in step a After the pH value of the ammonium sulfate solution was adjusted to neutral, 25ml of it was slowly added dropwise to the acetone reaction solution of cyanuric chloride, and crushed ice was added to maintain the reaction temperature at 0-5°C. 3 The pH value of the solution was controlled at 4, and a condensation intermedi...
Embodiment 3
[0038] a. Dissolve 15g of m-aminoacetanilide in 100ml of deionized water, slowly add 40ml of dimethyl sulfate to the aqueous solution of m-aminoacetanilide dropwise at 65°C and pH=7, react for 12 hours, cool to room temperature, add 10ml of concentrated sulfuric acid was deacetylated at 90°C for 1 hour to obtain the intermediate 3-amino-N,N,N-trimethylbenzyl ammonium sulfate solution, which was directly used in the next step without filtration;
[0039] b. Dissolve 2.77g of cyanuric chloride in 70ml of acetone, and add 10g of crushed ice to make a slurry, and use 2mol / L NaOH solution to dissolve the intermediate 3-amino-N,N,N-trimethylbenzene prepared in step a After the pH value of the ammonium sulfate solution was adjusted to neutral, 25ml of it was slowly added dropwise to the acetone reaction solution of cyanuric chloride, and crushed ice was added to maintain the reaction temperature at 0-5°C. 3 The pH value of the solution was controlled at 4, and a condensation intermed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com