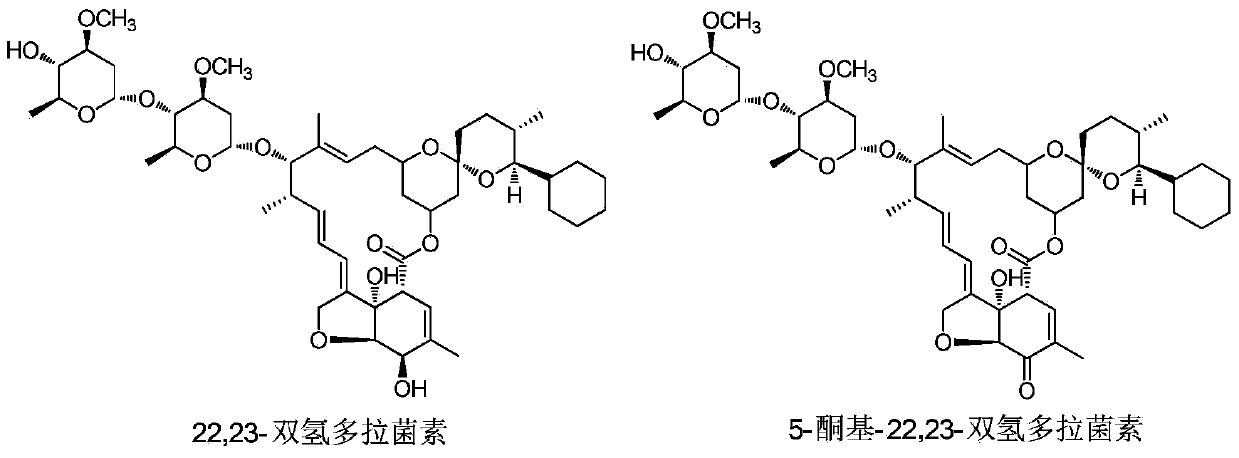
A genetically engineered bacterium producing 5-keto-22,23-dihydrodoramectin and its preparation method and application
A technology of dihydrodoramectin and genetically engineered bacteria, applied in the field of genetically engineered bacteria producing 5-keto-22,23-dihydrodoramectin and its preparation and application, can solve problems such as loss, Achieve the effects of improving production efficiency, reducing production costs and reducing negative impacts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
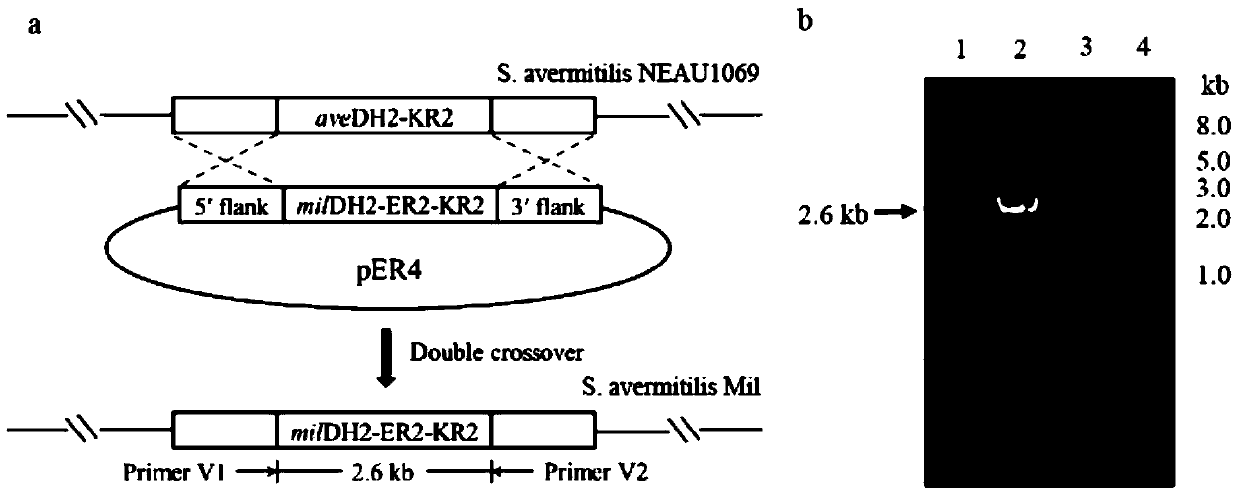
[0046] Embodiment 1, the construction of genetically engineered bacteria Streptomyces avermitilis Mil
[0047] method:
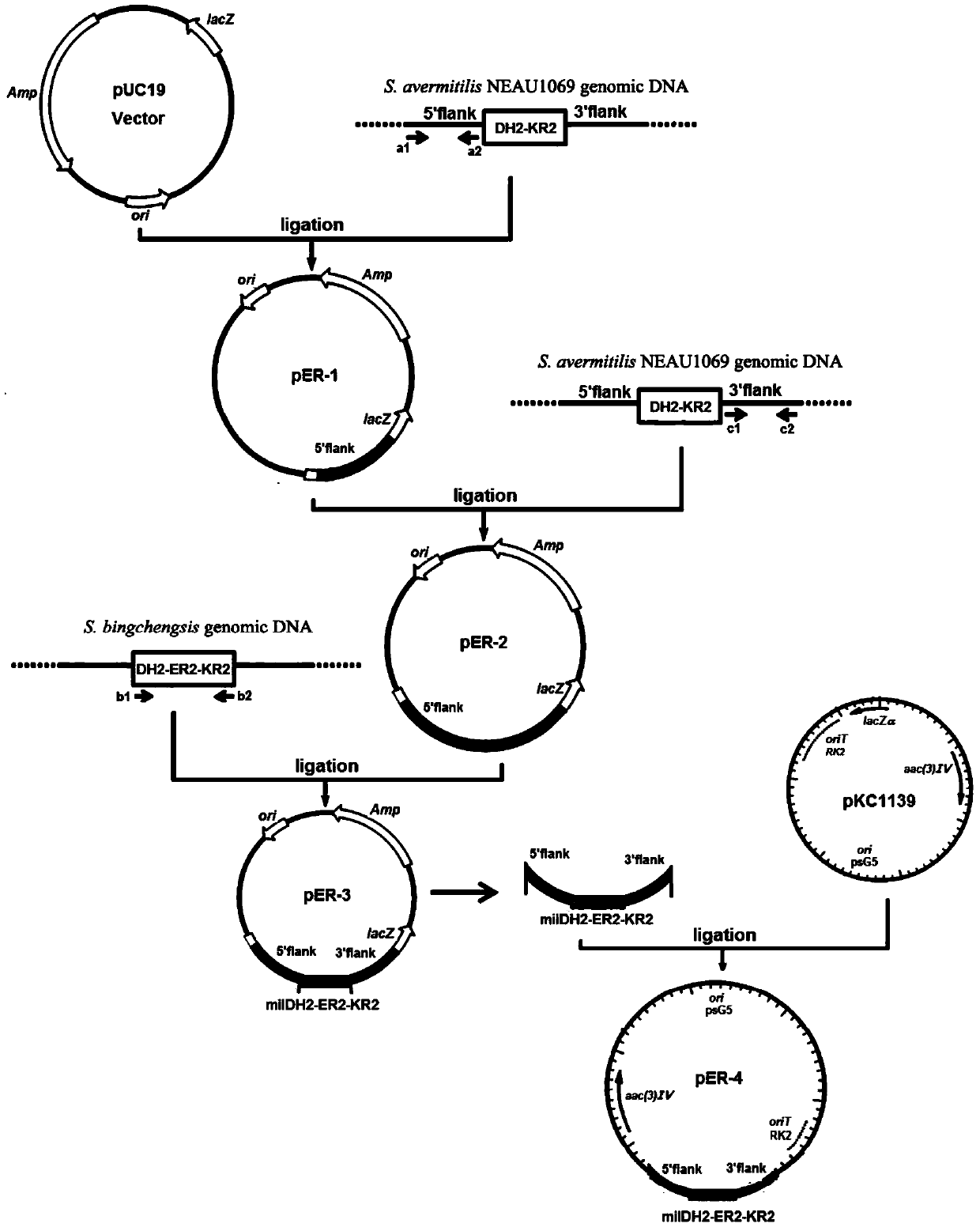
[0048] 1. Construction of the replacement plasmid pER-4
[0049] Using primer a1 (SEQ ID NO.1, 5'-CC AAGCTT CCGCATTCATCTGCTCCG-3', the underline is the HindIII site) and a2 (SEQ ID NO.2, 5'-GC TCTAGA CCGGCTGCTGACACGTTGC-3', the underline is the XbaI site) PCR amplified from the Streptomyces avermitilis NEAU1069 genome to obtain the homologous recombination left arm fragment of the DH2-KR2 gene in the avermectin biosynthesis gene cluster.
[0050] The total system of the PCR reaction is 25 μL, including 10×pfu Buffer 2.5 μL, dNTP 2 μL, Streptomycesavermitilis NEAU1069 genomic DNA 1 μL, primer a1 and primer a2 2 μL each, pfu DNA polymerase (5U / μL) 2 μL, DMSO 2.5 μL, water 6 μL . The reaction conditions are: 98°C for 1min; 98°C for 10s, 61.3°C for 30s, 72°C for 1min, 30cycles; 72°C for 10min. After the PCR product was loaded for electrophoresis, the targ...
Embodiment 2
[0061] Embodiment 2, the construction of genetically engineered bacteria Streptomyces avermitilis Mil-AveF
[0062] 1. Construction of knockout plasmid pER-aveF
[0063] Using primer aveF-F1 (SEQ ID NO.7, 5'-CCC AAGCTT GGGCCGAACTCTCGCTGTGCCGTG-3', the underline is the HindIII site) and aveF-R1 (SEQ ID NO.8, 5'-GC TCTAGA CGACATGAACCGCGCGATGCGCGA-3', the underline is the XbaI site) PCR amplified from the Streptomyces avermitilis NEAU1069 genome to obtain the homologous recombination left arm fragment of the aveF gene in the avermectin biosynthesis gene cluster.
[0064] The total system of the PCR reaction is 25 μL, including 10×pfu Buffer 2.5 μL, dNTP 2 μL, Streptomycesavermitilis NEAU1069 genomic DNA 1 μL, primer aveF-F1 and primer aveF-R1 2 μL each, pfu DNA polymerase (5U / μL) 2 μL, DMSO 2.5 μL, water 6 μL. The reaction conditions are: 98°C for 1min; 98°C for 30s, 56°C for 30s, 72°C for 1min, 30cycles; 72°C for 10min. After the PCR product was loaded for electrophoresis,...
Embodiment 3
[0073] Example 3, Fermentation of genetically engineered bacteria Streptomyces avermitilis Mil and Streptomycesavermitilis Mil-AveF and MS identification of target compounds
[0074] Pick the original strain Streptomyces avermitilis NEAU1069, the genetically engineered strains Streptomyces avermitilis Mil and Streptomyces avermitilis Mil-AveF, respectively, and culture them on Gaoshi No. 5.0, soy peptone 2.0, CoCl 2 ·6H 2 O 0.01 (g / 1000mL), pH7.0), and then cultured at 28°C and 250r / min for 42h. Get 2.0mL of seed culture solution and transfer to 25mL of fermentation medium (glucose 0.5%, cornstarch 10%, peptone 1%, cottonseed meal 1%, NaCl 0.1%, K 2 HPO 4 0.2%, MgSO 4 ·7H 2 O 0.1%, CaCO 3 0.7%, pH7.2), 28°C, 250r / min for 10 days. At 24h and 168h of fermentation, cyclohexanecarboxylic acid with a final concentration of 100mg / L and 60mg / L was added to the fermentation medium, respectively.
[0075] After the fermentation, the fermented liquid was mixed with an equal vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com