Treatment method for waste lithium-ion battery
A technology of lithium ion battery and treatment method, applied in the field of waste lithium ion battery treatment, can solve the problems of online production of active substances, neglect of recycling treatment, secondary environmental pollution, etc., so as to increase battery recycling treatment and shorten production process , the effect of strong applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
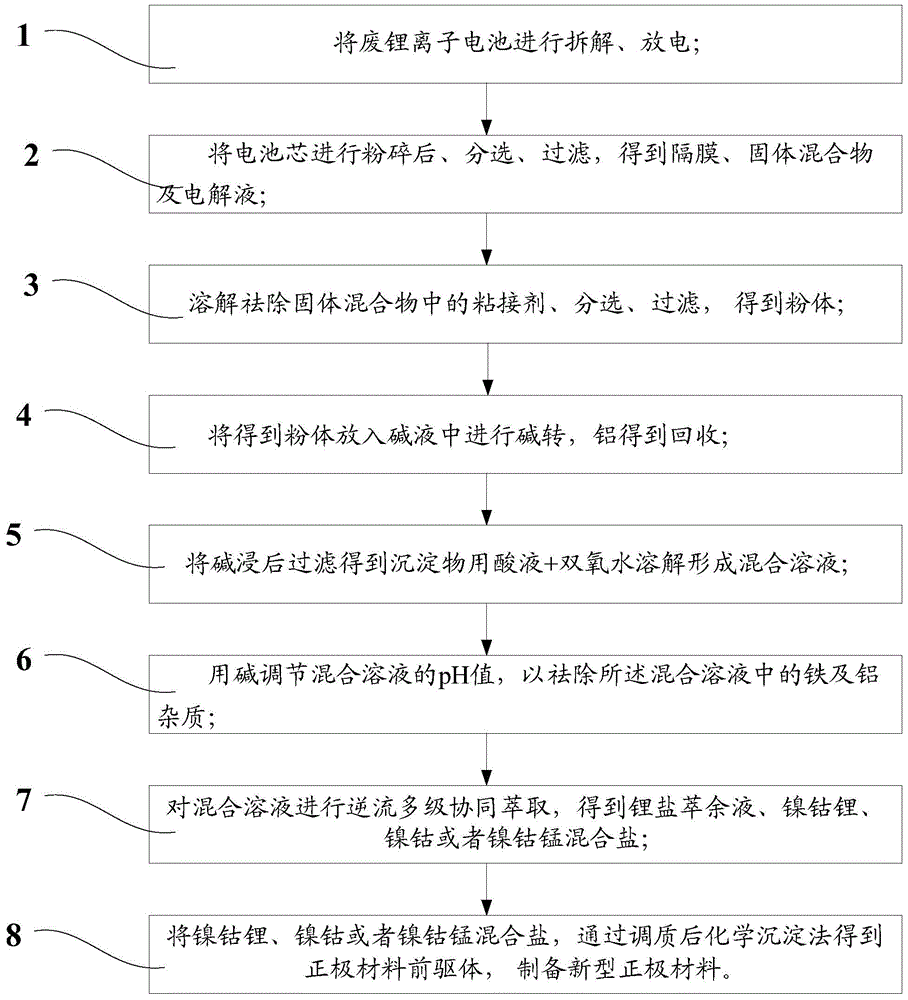
[0033] Specific embodiments of the present invention will be described in detail below in conjunction with the accompanying drawings. It should be understood that the specific embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
[0034] like figure 1 as shown, figure 1 A flow chart of a method for treating a spent lithium-ion battery provided by an embodiment of the present invention.
[0035] The embodiment of the present invention provides a kind of processing method of waste lithium ion battery, and this method comprises the following steps:
[0036] Disassemble the waste lithium-ion battery to obtain the shell, tabs and battery core;
[0037] Pulverize the battery core to obtain a solid mixture, electrolyte, and diaphragm;
[0038] Sorting and filtering the obtained solid mixture, electrolyte and diaphragm to obtain a solid mixture;
[0039] Dissolving and removing the binder in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com