Pharmaceutical composition containing PEGylated asparaginase and preparation method thereof
An asparaginase and pegylation technology, which is used in drug combinations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of short validity period, unexpected temperature fluctuations, and high transportation costs, and achieves extended validity period, Not easy to fall off, strong binding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
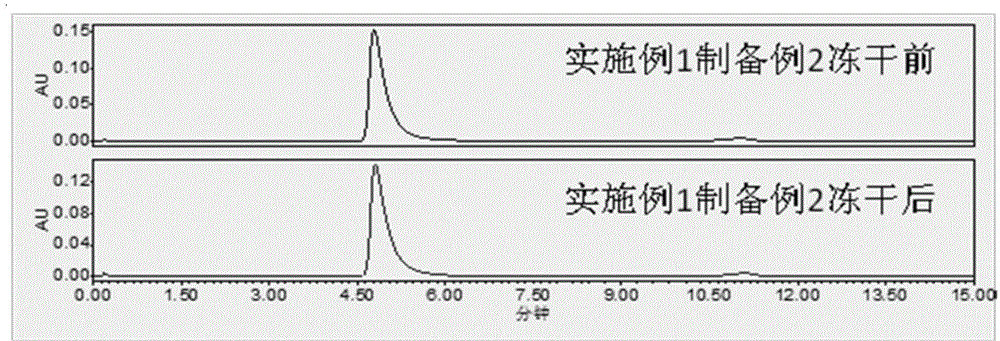
[0070] Example 1 Pegylated asparaginase prescription for injection (both in mass percent) and its preparation process
preparation example 1
[0072]
[0073]
[0074] Preparation Process:
[0075] Solution preparation: Prepare a 50mM phosphate buffer solution containing 5% sorbitol, check the pH value at 6.0-8.0 and cool down to below 25°C, dilute pegylated asparaginase with the buffer solution prepared above, and detect the intermediate . The medicinal liquid is sterilized and filtered through a 0.2um microporous filter, filled with a plug and capped, and the water needle is obtained. If it is to be made into freeze-dried powder injection, after filling, half-tighten the stopper, put it into a freeze-drying box, and carry out the freeze-drying process.
[0076] Freeze drying process:
[0077] Pre-freezing stage: Pre-freezing adopts full-speed cooling of the plate layer, and the product temperature drops to -60°C, and keeps for 3 hours to completely freeze the sample.
[0078] Sublimation stage: turn on the vacuum pump, pre-evacuate to 0.40mbar, start sublimation, heat the separator at a rate of 15°C per ho...
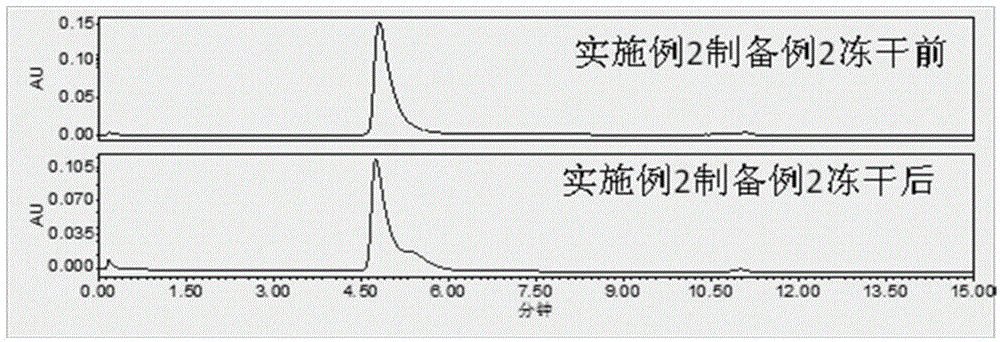
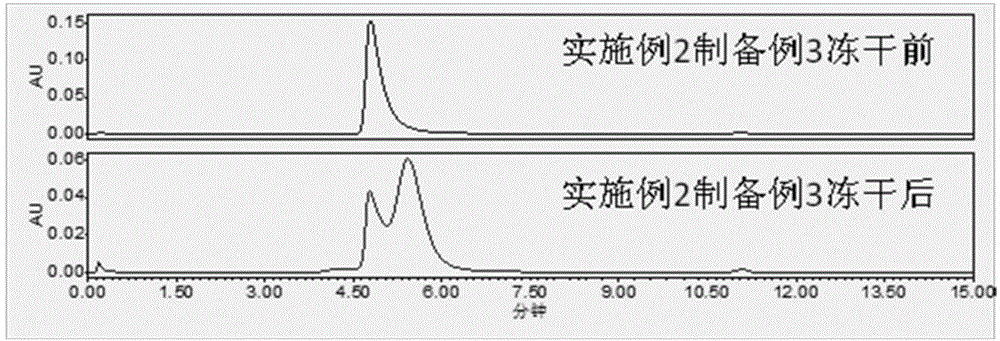
preparation example 2
[0081]
[0082] Preparation Process:
[0083] Solution preparation: prepare a phosphate buffer solution containing 3% sorbitol 100mM, check the pH value at 6.0-8.0 and cool down to below 25°C, dilute pegylated asparaginase with the buffer solution prepared above, and detect the intermediate . The medicinal liquid is sterilized and filtered through a 0.2um microporous filter, filled with a plug and capped, and the water needle is obtained. If it is to be made into freeze-dried powder injection, after filling, half-tighten the stopper, put it into a freeze-drying box, and carry out the freeze-drying process.
[0084] Freeze drying process:
[0085]Pre-freezing stage: Pre-freezing adopts full-speed cooling of the plate layer, and the product temperature drops to -60°C, and keeps for 3 hours to completely freeze the sample.
[0086] Sublimation stage: turn on the vacuum pump, pre-evacuate to 0.40mbar, start sublimation, heat the separator at a rate of 20°C per hour, control ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com