4-amino alkoxy-3-methoxy cinnamic acid benzamide compounds as well as preparation methods and applications thereof
A technology of cinnamic acid benzamide and methoxycinnamic acid, which is applied in the field of preparation of 4-aminoalkoxy-3-methoxycinnamic acid benzamide compounds, can solve the problem of multiple toxic and side effects, single action target, AD Patients with poor long-term curative effect and other problems, to achieve the effect of cell damage protection and cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
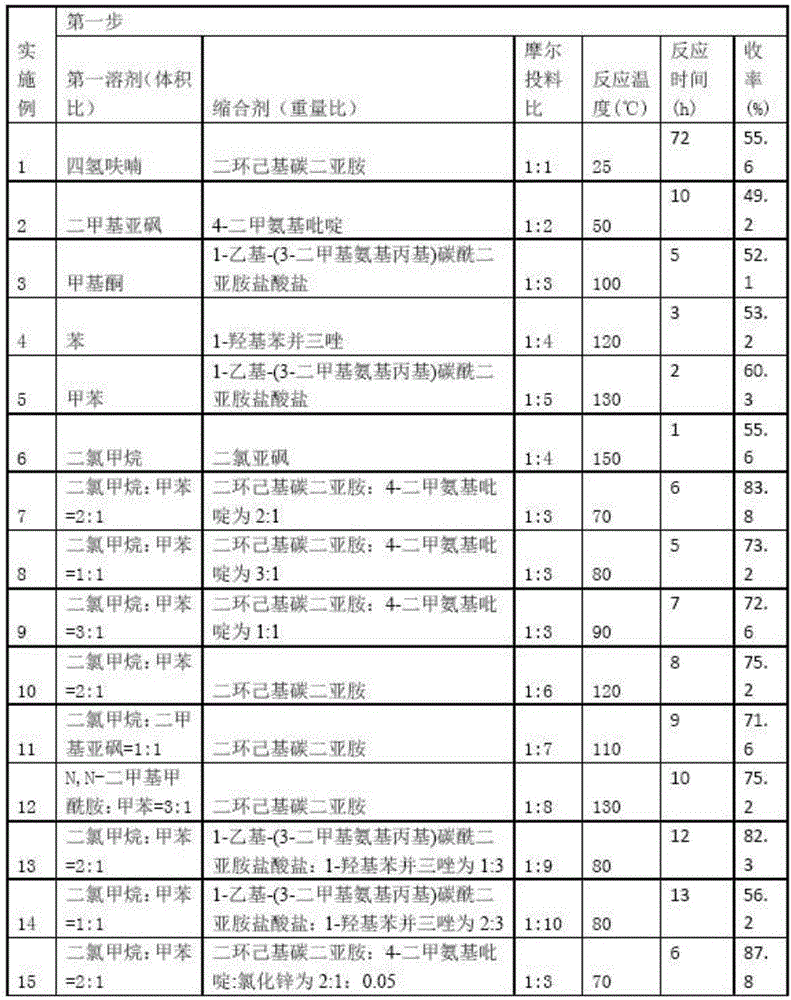
Embodiment 1-15
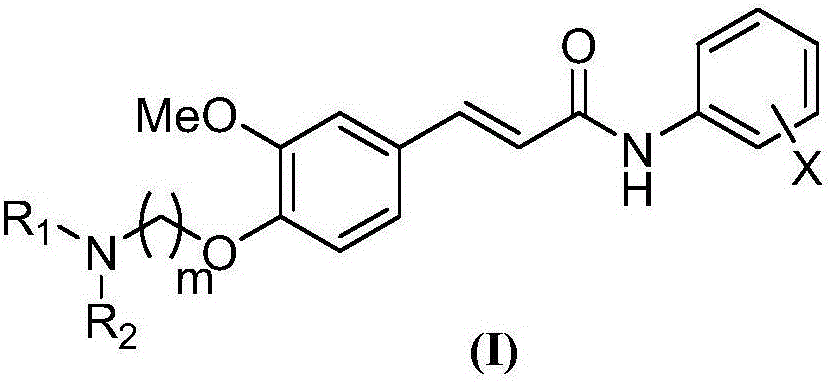
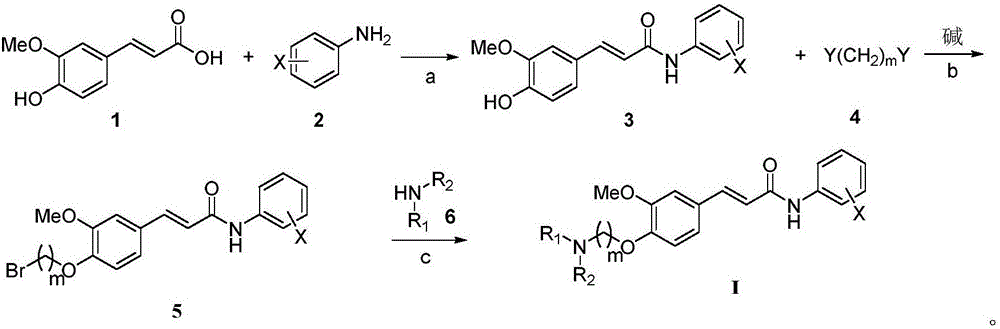
[0051] A preparation method of 4-aminoalkoxy-3-methoxycinnamic acid benzamide compound, comprising the following steps:
[0052] The first step, add ferulic acid, the first solvent, condensing agent and aniline in reaction bottle, stir reaction, reaction progress is tracked with TLC; Wash with saturated aqueous sodium carbonate and saturated aqueous sodium chloride, dry the organic layer over anhydrous sodium sulfate and filter, distill off the solvent under reduced pressure, and purify the residue by column chromatography (eluent: dichloromethane: methanol = 30: 1v / v), get corresponding ferulic acid benzamide compounds;
[0053] Second step, the above-mentioned ferulic acid benzamide compounds are dissolved in the second solvent, add the used alkali and dibromoethane under the first basic condition, heat up and reflux and stir the reaction, the reaction process is followed by TLC, after the reaction finishes, press The solvent and excess dibromoethane were evaporated, and th...
Embodiment 16
[0066] The specific process conditions are the same as in Example 7, and the difference is the same as that of investigating different substituents. The specific substituents are shown in Table 4. The obtained 4-aminoalkoxy-3-methoxycinnamic acid benzamides have the same chemical structure. through 1 H-NMR, 13 Confirmed by C-NMR and ESI-MS.
[0067] Table 4 Different Substituent Experiments
[0068]
[0069]
[0070]
[0071]
[0072]
Embodiment 17
[0074] A preparation method of a pharmaceutically acceptable salt of a 4-aminoalkoxy-3-methoxycinnamic acid benzamide compound, comprising the following steps:
[0075] Take the 4-aminoalkoxy-3-methoxycinnamic acid benzamide compound and acetone respectively in Table 4 and stir evenly, then add the corresponding acid, heat up and reflux and stir for 15-30 minutes, and cool to room temperature after the reaction. The solvent was distilled off under reduced pressure, the residue was recrystallized with acetone, and the precipitated solid was filtered to obtain a pharmaceutically acceptable salt of 4-aminoalkoxy-3-methoxycinnamic acid benzamide compound. 1 HNR, 13 Confirmed by CNMR and ESI-MS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com