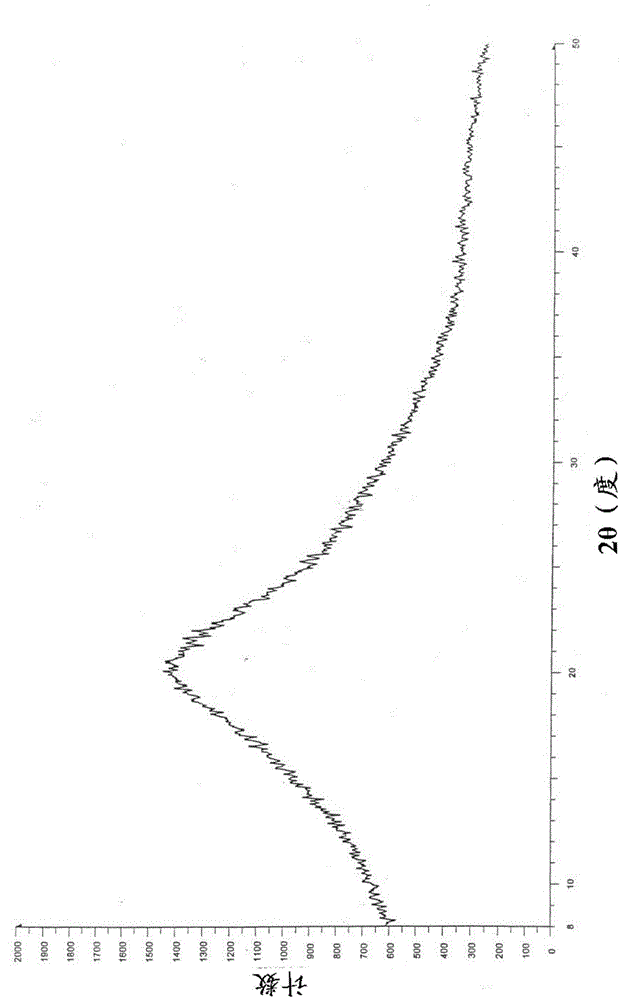
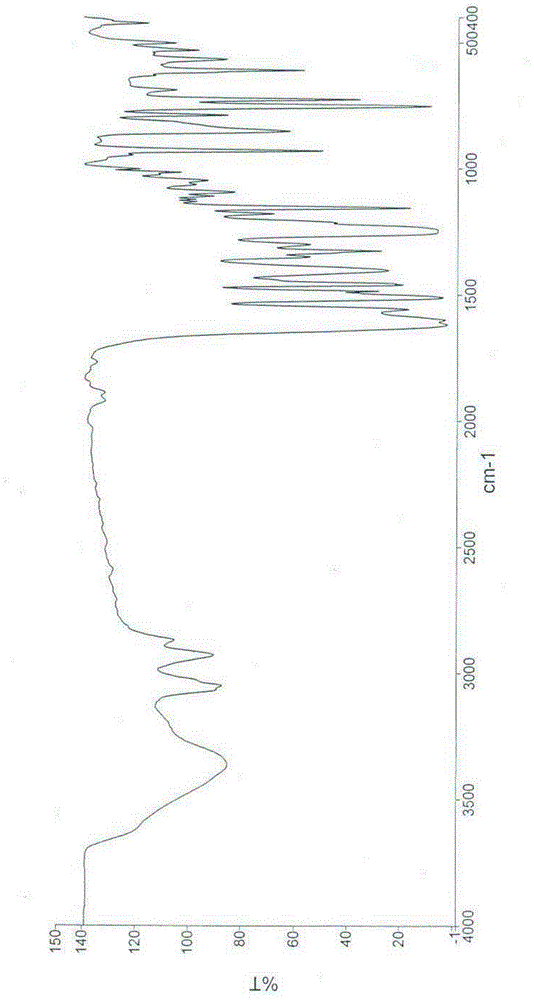
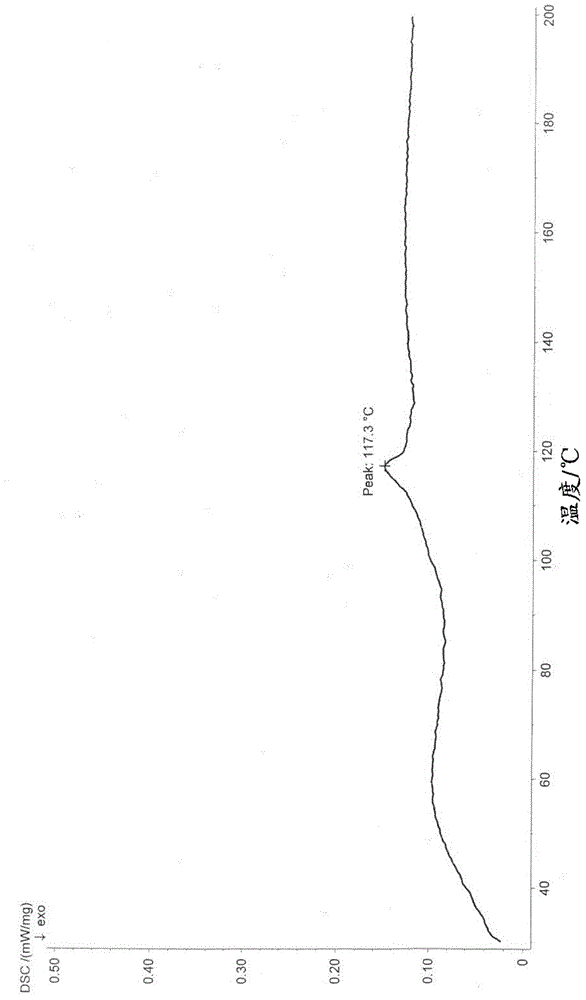
Salts of phenylalanine compound and amorphous form of salts
A compound and amorphous technology, applied in the field of drugs for diseases, salts of phenylalanine compounds, and amorphous forms, can solve problems affecting drug safety and effectiveness, low bioavailability, easy decomposition, etc. To achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Sodium 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionate preparation of
[0042]
[0043] Add 487.4g (12.18mol) of NaOH and 10.85kg of anhydrous methanol into a 50L plastic bucket, stir and dissolve at room temperature. Add 184kg tetrahydrofuran and 6.90kg (12.06mol) 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2- (9H-carbazol-9-yl)ethoxy)phenyl)propionic acid was stirred and dissolved at room temperature, and the above-mentioned methanol solution in which sodium hydroxide was dissolved was added to the reaction kettle, and stirring was continued for 30 minutes after the addition was completed. Concentrate in vacuo to remove tetrahydrofuran and methanol to obtain a concentrate. Dissolve the concentrate in 18.29kg of dichloromethane, add it dropwise to 149kg of isopropyl ether at room temperature with stirring, centrifuge, collect the solid, and dry it in vacuum at 105°C for 3 hours to obtain 2-(2-(4-fluorobenzyl Sodium ac...
Embodiment 2
[0045] Example 2: Potassium 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionate preparation of
[0046]
[0047] Add 0.495g (8.84mmol) KOH and 10mL anhydrous methanol into a 100mL single-necked bottle, stir at room temperature to dissolve. Add 5.00g (8.74mmol) 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy Base) phenyl) propionic acid and 150ml tetrahydrofuran, stirring at room temperature to dissolve, adding the above-mentioned methanol solution in which potassium hydroxide was dissolved, and continuing to stir for 30 minutes after the addition. Concentrate in vacuo to remove tetrahydrofuran and methanol to obtain a concentrate. Dissolve the concentrate in 10 mL of dichloromethane, add dropwise to 150 mL of anhydrous diethyl ether at room temperature with stirring, filter, collect the solid, and dry it in vacuum at 60°C for 10 h to obtain 2-(2-(4-fluorobenzoyl) Potassium phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy...
Embodiment 3
[0049] Embodiment 3: stability test
[0050] 1. Test sample
[0051] 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propanoic acid: according to Chinese patent application 03126974.5 was prepared in Example 20, batch number: T1263-07-12-02, purity (HPLC): 99.34%.
[0052]Sodium 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionate amorphous form: Prepared according to the preparation method of Example 1 of the present invention, batch number: 20130606, purity (HPLC): 99.61%.
[0053] Potassium 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionate amorphous form: Prepared according to the preparation method of Example 2 of the present invention, batch number: 20130619, purity (HPLC): 99.52%.
[0054] 2. Test plan
[0055] According to the "Chinese Pharmacopoeia" 2010 edition two appendix XIXC to carry out the high temperature (60 ℃), high humidity (90%) and strong light (45001x) inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com