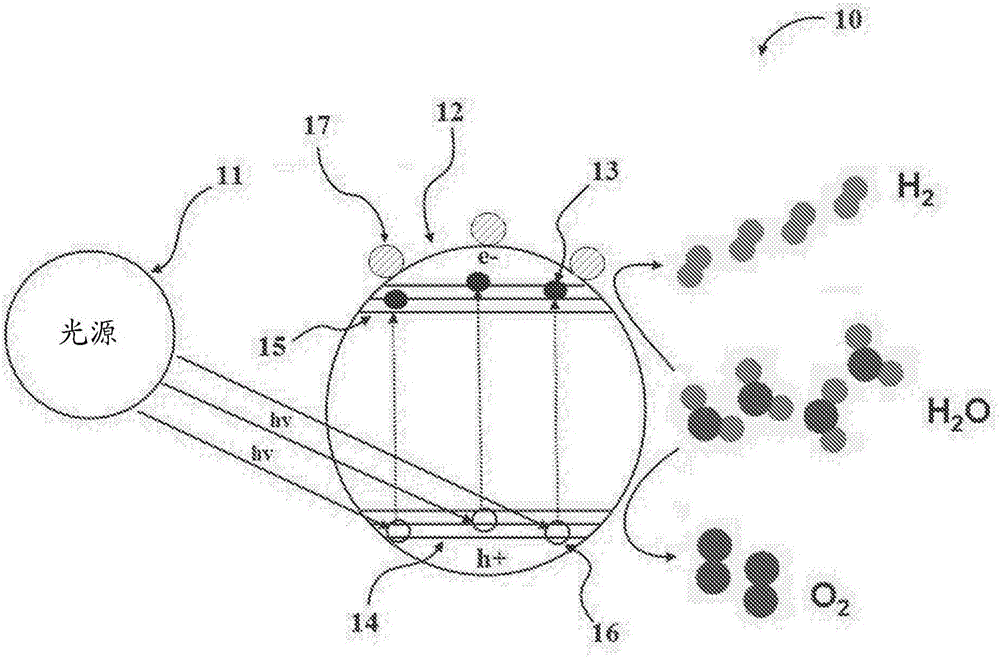
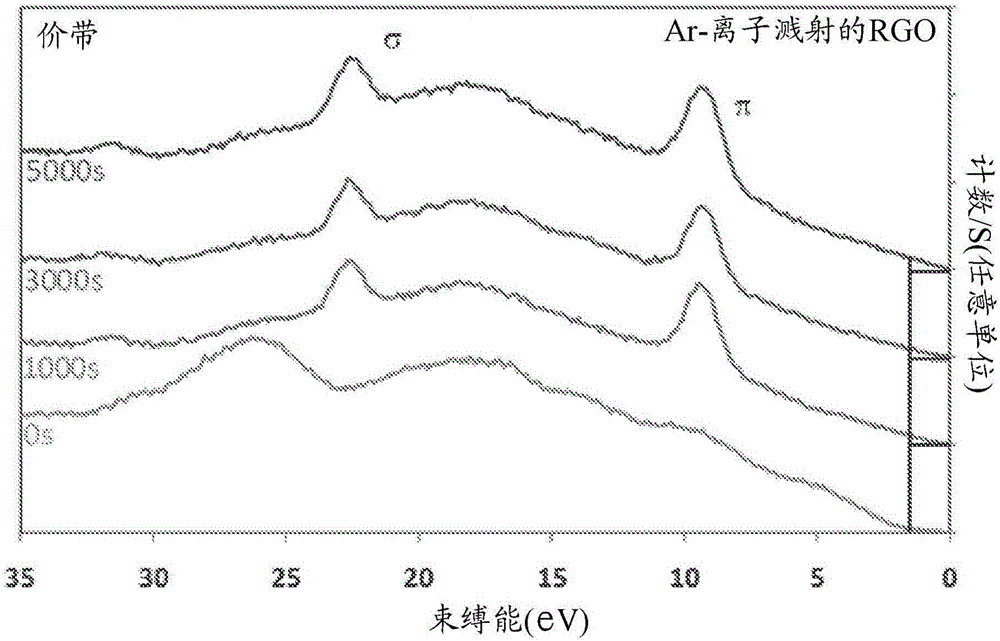
Hydrogen production from water using photocatalysts comprising metal oxides and graphene nanoparticles
A photocatalyst, nanoparticle technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, oxygen/ozone/oxide/hydroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (Materials and Methods for Preparation, Testing and Characterization of Photocatalysts)
[0040] Synthesis of reduced graphene oxide:
[0041] Graphene oxide (GO) was prepared from graphite using a modified Hummers method (Hummers & Offeman, 1958). In a dry 500 mL round bottom flask equipped with a magnetic stirrer, graphite powder (1 g), sodium nitrate (1 g, 11.76 mmol) and sulfuric acid (46 mL) were combined and stirred in an ice bath. To the resulting reaction mixture was slowly added KMnO 4 (6 g, 37.96 mmol). After mixing, the reaction flask was transferred to an oil bath and stirred vigorously at 40 °C for 1 h. To the resulting brown paste was added 80ml of water, and the slurry was stirred for a further 1 h while raising the temperature to 90°C. Finally, 200 mL of water was added, followed by the slow addition of 6 mL of H 2 o 2 (30%), the color of the solution turned from dark brown to brownish yellow. The product was filtered off (while hot), washed wit...
Embodiment 2
[0050] (water splitting reaction)
[0051] The prepared catalyst from Example 1 (20 mg, powder) was charged into a batch reactor. The catalyst was subsequently reduced at 300°C for one hour. The reactor was purged with nitrogen for 30 min. Water (25ml) was then injected into the reactor. The mixture was stirred under UV-irradiation. Gas samples were collected using a syringe at different time intervals and analyzed by using GC-TCD equipped with a PorapakQ column.
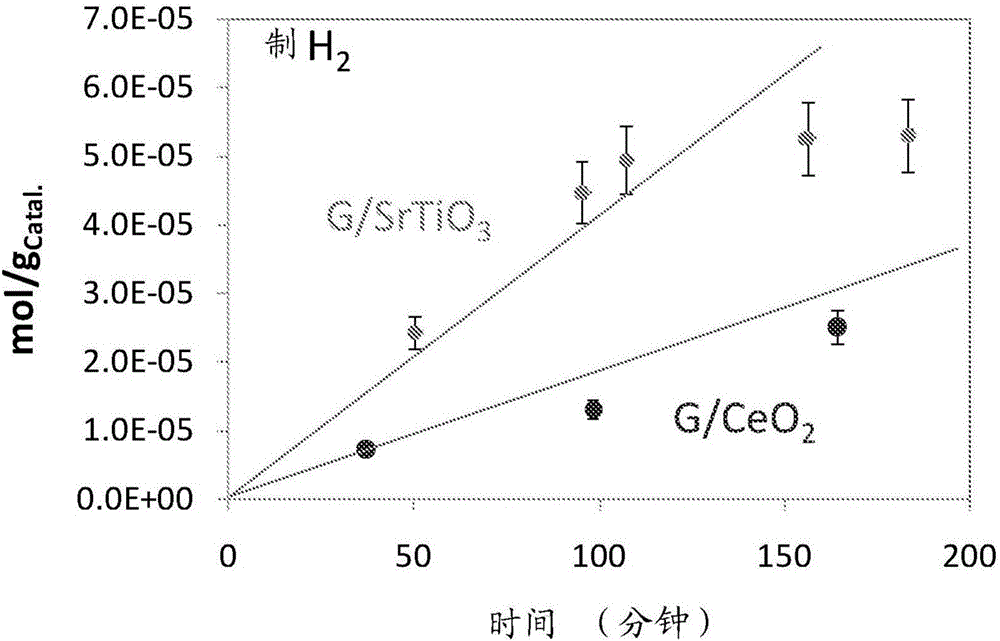
[0052] image 3 shows the use of graphene / SrTiO 3 and graphene / CeO 2 Results of UV-excitation experiments of catalysts. In graphene / SrTiO 3 In the case of , hydrogen production appears linear until about 100 min of the reaction, after which the rate of production slows down significantly. Considering the SrTiO used in this work 3 surface area, which is about 3m 2 / g and is approximately equal to 2×10 in this surface 19 O atoms, the total hydrogen concentration was found to be 3×10 per gCatal. 19 molecul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com