Method for preparing hydrogen through hydrogen iodide catalysis and decomposition
A technology of catalytic decomposition and hydrogen iodide, which is applied in the direction of iodine/hydrogen iodide, chemical instruments and methods, physical/chemical process catalysts, etc. Poor performance and other problems, to achieve the effect of wide reaction temperature range and pressure range, good catalyst economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
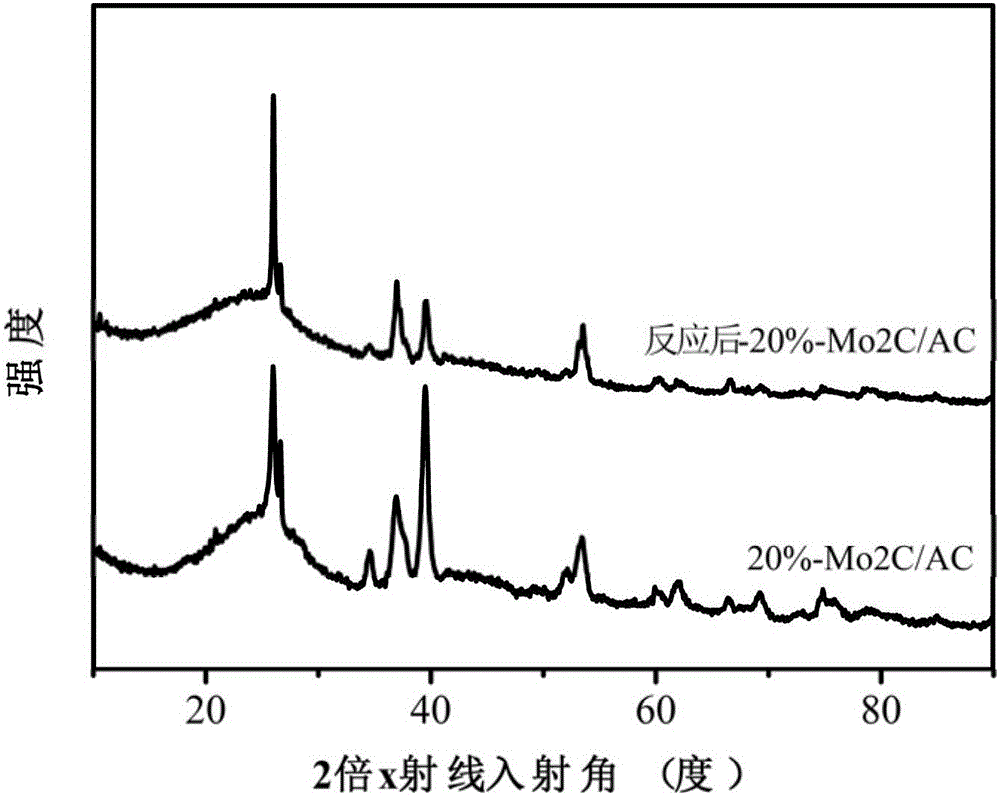
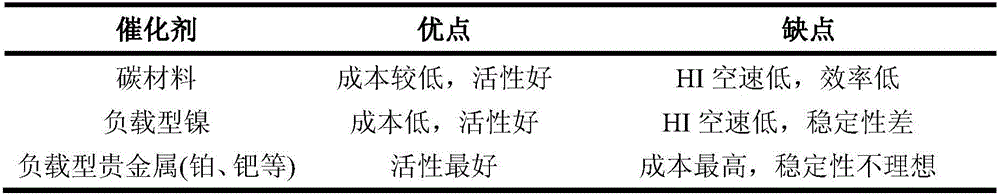
Image
Examples
Embodiment 1
[0023] 0.5 g of activated carbon (AC) loaded molybdenum carbide 5% Mo 2 The C / AC catalyst is loaded into a tubular fixed-bed reactor for the decomposition of hydrogen iodide, and the reaction temperature is controlled at 500° C. Hydroiodic acid (analytical pure hydroiodic acid, HI content ≥ 57%, Rizhao Lidex Chemical Co., Ltd.) The feed rate is 1.0ml / min, and the reaction pressure is normal pressure. Utilize sodium hydroxide standard solution to titrate the H in hydroiodic acid before and after the reaction + concentration to calculate the conversion rate of reaction, hydrogen iodide conversion rate is 22%, close to the thermodynamic equilibrium conversion rate under this condition (at 1atm, under 500 ℃ of reaction conditions, hydrogen iodide decomposition equilibrium conversion rate is about 23%).
Embodiment 2
[0027] 0.2 g of molybdenum carbide supported on activated carbon (AC) 1% Mo 2 The C / AC catalyst was loaded into a tubular fixed-bed reactor for hydrogen iodide decomposition, the reaction temperature was controlled at 500° C., the feed rate of hydroiodic acid was 1.0 ml / min, and the reaction pressure was normal pressure. Utilize sodium hydroxide standard solution to titrate the H in hydroiodic acid before and after the reaction + Concentration to calculate the conversion rate of reaction, hydrogen iodide conversion rate is 7%.
Embodiment 3
[0032] 0.2 g of activated carbon (AC) loaded molybdenum carbide 5% Mo 2The C / AC catalyst was loaded into a tubular fixed-bed reactor for hydrogen iodide decomposition, the reaction temperature was controlled at 500° C., the feed rate of hydroiodic acid was 1.0 ml / min, and the reaction pressure was normal pressure. Utilize sodium hydroxide standard solution to titrate the H in hydroiodic acid before and after the reaction + Concentration to calculate the conversion rate of reaction, hydrogen iodide conversion rate is 9.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com