Preparation method of notoginseng raw powder tablet
The technology of Panax notoginseng and powder tablets is applied in the field of preparation of the original powder tablets of Panax notoginseng, which can solve the problems that the effective components of Panax notoginseng are not easily leached, the effective components and the efficacy of drugs are reduced, and the health-care effect cannot be achieved, and the number of manual turning of materials can be reduced. , Improve health care function, the effect of uniform and stable heating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
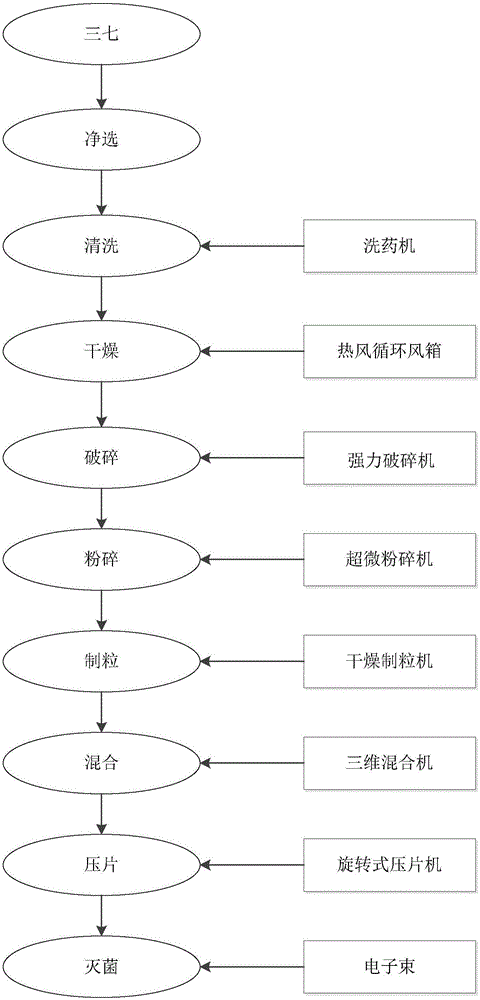
[0053] Such as figure 1 Shown, a kind of preparation method of Radix Notoginseng original powder sheet, at first selects ginseng: selects Radix Notoginseng with thick feet, solid body, no disease scar, no disability, and beautiful body as processing raw material. Then the steps are as follows:
[0054] Step 1. Clean selection: put Panax notoginseng on the clean selection operation table, and pick out impurities, foreign objects, insects, mildew and non-medicinal parts;
[0055] Step 2. Cleaning: Put the qualified Panax notoginseng into the air bubble washing machine, wash the raw materials with drinking water until the surface is clean and free of mud, and brush away the debris in the wrinkles and between the forks until it is clean;
[0056] Step 3. Drying: Put the cleaned notoginseng into a stainless steel drying tray. The thickness of the notoginseng tray is 25-35mm, preferably no more than 30mm. Then put the stainless steel drying tray into a hot air circulation air box f...
Embodiment 2
[0064] The difference between this example and Example 1 is: in the drying step of step 3, the drying temperature is controlled at 50° C., the drying time is 12 minutes, and the water content of Panax notoginseng is controlled to be less than 8%. The lower the water content, the easier it is to store.
Embodiment 3
[0066] The difference between this embodiment and Example 1 is: in the crushing step of step 5, the freezing medium is a type A freezing liquid, and the fructose syrup used is F42 fructose syrup and F55 fructose syrup, and the mass percentage content of the two is 60%. and 40%. Control the temperature of the outer jacket of the pulverizing chamber of the superfine pulverizer at -40°C for low-temperature pulverization for 20 minutes, and pulverize into fine powder with an average particle size of 5 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com