Method for catalytically synthesizing vitamin K2 through NovQ aromatic isopentenyl transferase
A technology for prenyl and vitamin, which is applied in the field of aromatic prenyl transferase catalyzed synthesis of fine chemicals, can solve the problems of difficulty in meeting large-scale production needs, complex purification process, low synthesis efficiency, etc., and achieves the production process. The effect of green environmental protection, simple production process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
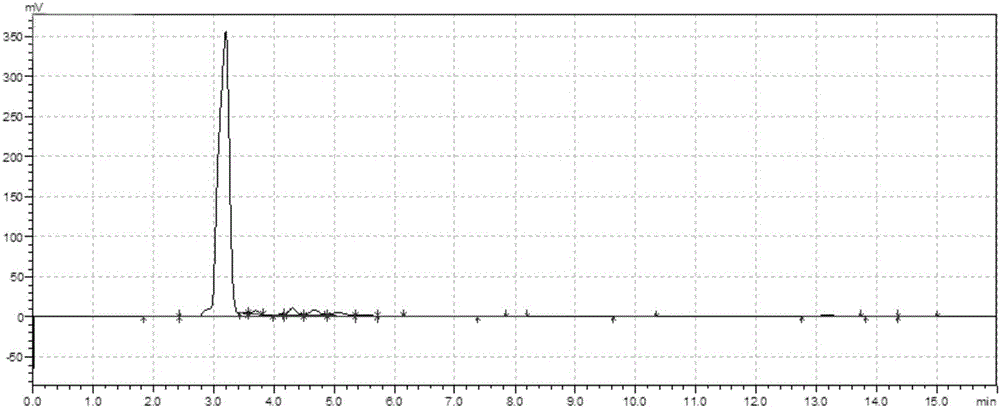
[0029] 5.17g (30mmol) vitamin K 3 Dissolve in 40mL aqueous ethanol (1:1, v / v), add 1.13g (30mmol) NaBH in portions 4 , at N 2 Under protection, control the temperature not to exceed 50°C and stir the reaction for 12 hours until the system becomes a light green transparent liquid. After neutralizing to pH 4.0 with 100mmol / L dilute hydrochloric acid, extract the product three times with 120mL ethyl acetate, concentrate the extract to about 8mL under nitrogen protection, freeze and crystallize at -80°C, filter to retain the crystals, and concentrate the supernatant to 1.6mL, freeze and crystallize at -80°C, filter, combine the filtered crystals twice to obtain 4.73g vitamin K 3 Hydroquinone, yield 89.5%. After being oxidized in the air, it is detected by HPLC, and its peak characteristics are as follows: figure 1 Shown (peak time 3.19min, detector A248nm).
Embodiment 2
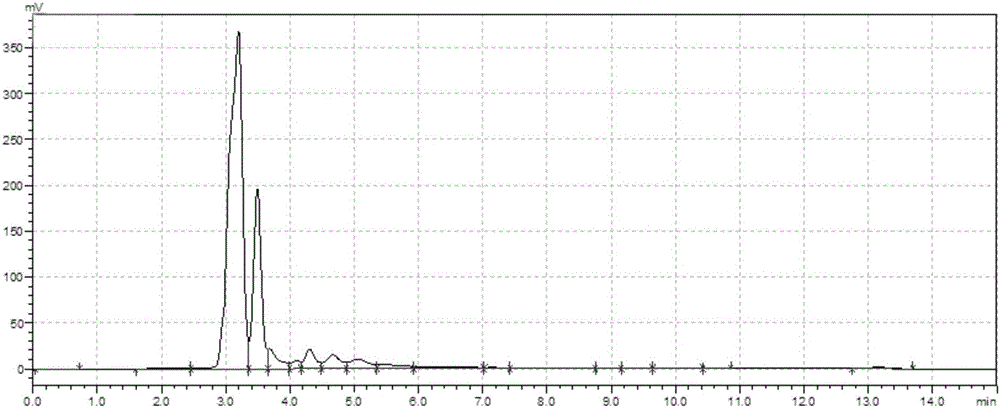
[0031] 107.44g (780mmol) vitamin K 3 Dissolve in 800mL aqueous ethanol (3:1, v / v), add 45.40g (1200mmol) NaBH in portions 4 , at N 2 Under protection, the temperature was controlled not to exceed 50°C, and the reaction was stirred for 6 hours until the system became a light green transparent liquid. After neutralizing to pH 4.0 with 100mmol / L dilute hydrochloric acid, the product was extracted three times with 2.4L ethyl acetate, and the extract was placed in N 2 Concentrate to about 0.177L under protection, freeze and crystallize at -20°C, filter to retain the crystals, concentrate the supernatant to about 0.039L, freeze and crystallize at -20°C, filter, combine the crystals filtered twice to obtain 96.07g of vitamin K 3 Hydroquinone, yield 90.9%.
Embodiment 3
[0033] 68.87g (400mmol) vitamin K 3 Dissolve in 500mL ethanol aqueous solution (2:1, v / v), add 11.35g (300mmol) NaBH 4 , at N 2 Under protection, the temperature was controlled not to exceed 50°C, and the reaction was stirred for 18 hours until the system became a light green transparent liquid. After being neutralized to pH 4.0 with 100mmol / L dilute hydrochloric acid, the product was extracted three times with 1500mL ethyl acetate, and the extract was placed in N 2Concentrate to about 91mL under protection, freeze and crystallize at -50°C, filter to retain the crystals, concentrate the supernatant to about 16.5mL, freeze and crystallize at -50°C, filter, combine the crystals filtered twice to obtain 57.85g of vitamin K 3 Hydroquinone, yield 82.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com