Ferric citrate for reducing cardiac failure in chronic kidney disease patients
A chronic kidney disease, iron citrate technology, applied in metabolic diseases, urinary system diseases, organic active ingredients, etc., can solve the lack of dialysis center infrastructure, poor intravenous iron administration equipment, and CKD iron deficiency patients. Intravenous iron therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
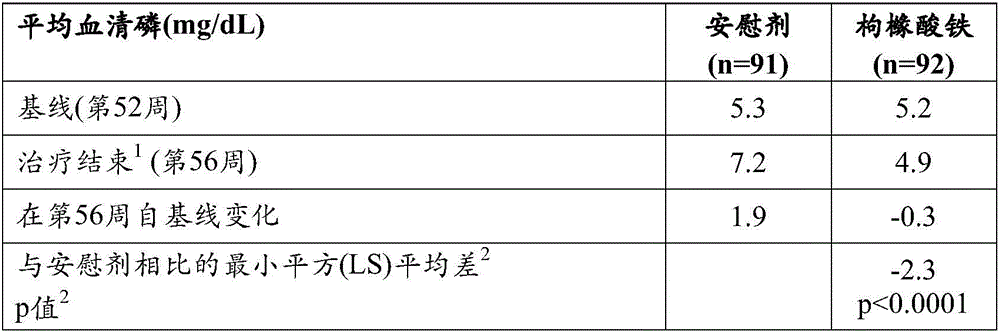
[0316] Phase 3 58-week trial of ferric citrate as a phosphate binder in patients on dialysis with end-stage renal disease (ESRD)
[0317] The main objectives of the trial are as follows:
[0318] 1. To determine the long-term safety of KRX-0502 (ferric citrate) up to twelve (12) caplets / day over 52 weeks in patients with end-stage renal disease undergoing hemodialysis or peritoneal dialysis.
[0319] 2. To determine the efficacy of KRX-0502 (ferric citrate) during the 4-week randomized open-label placebo-controlled efficacy evaluation period.
[0320] Research rationale
[0321] A previous clinical trial demonstrated the ability of ferric citrate to reduce serum phosphorus levels in ESRD patients undergoing thrice-weekly hemodialysis. These trials used a maximum of about 12 g / day ferric citrate for 4 weeks.
[0322] This clinical trial measured ferric citrate during a 56-week treatment period when compared with placebo during a 52-week safety evaluation period and a randomi...
Embodiment 2
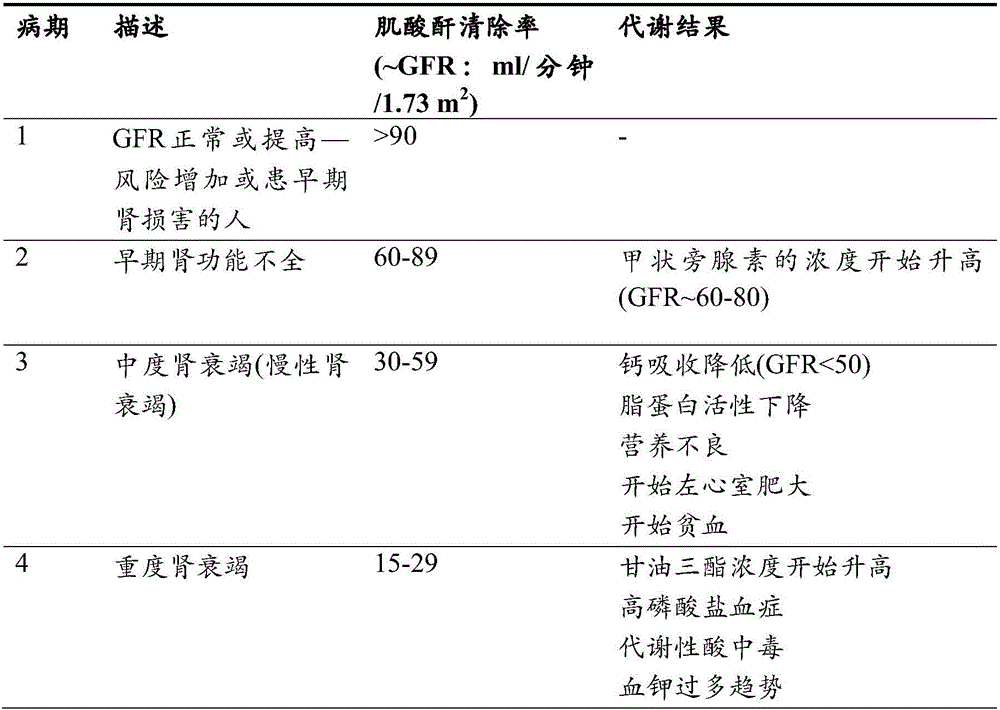
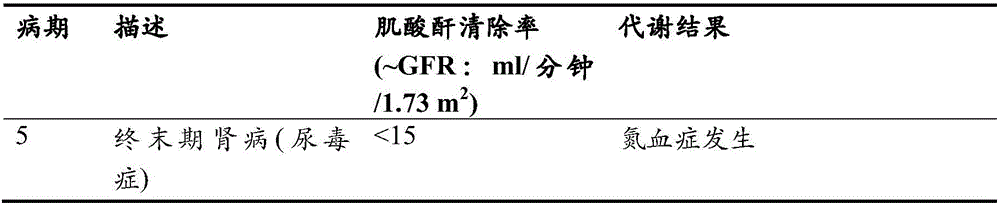
[0484] Study of KRX-0502 (ferric citrate) in the management of serum phosphorus and iron deficiency in anemic subjects with stage III-V chronic kidney disease not on dialysis
[0485] A phase 2 proof-of-concept multicenter randomized placebo-controlled open-label clinical trial was conducted.
[0486] The study lasts approximately 5-7 months with approximately 8-12 weeks allocated for subject screening, 2 weeks for subjects to wash off their existing phosphate binders (if taking), and 12 weeks allocated for administration of study drug For treatment, the research drug was ferric citrate or placebo disclosed herein. For the purposes of this example, the ferric citrate disclosed herein is referred to as KRX-0502 (ferric citrate).
[0487]The objective of the study was to determine the efficacy and safety of KRX-0502 (ferric citrate) in the management of serum phosphorus and iron deficiency in anemic subjects with non-dialysis-dependent stage III-V chronic kidney disease (CKD). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com