Monovalent blood brain barrier shuttle modules
A blood-brain barrier, monovalent technology, used in medical preparations with non-active ingredients, antibodies, targeting specific cell fusion, etc., can solve problems such as low antibody concentration and limited pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
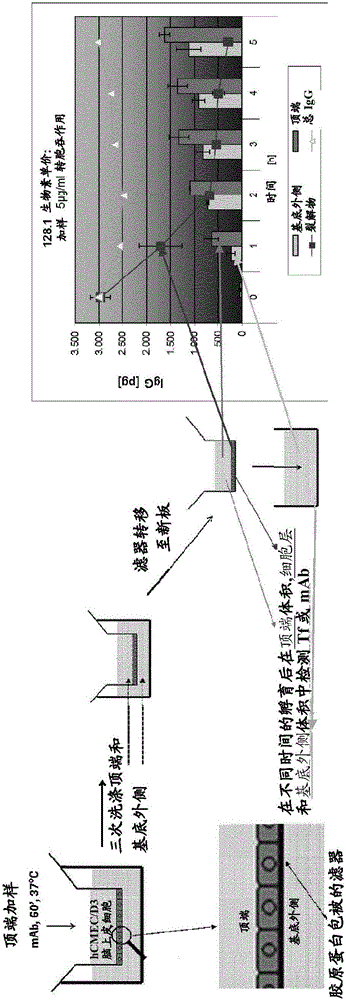
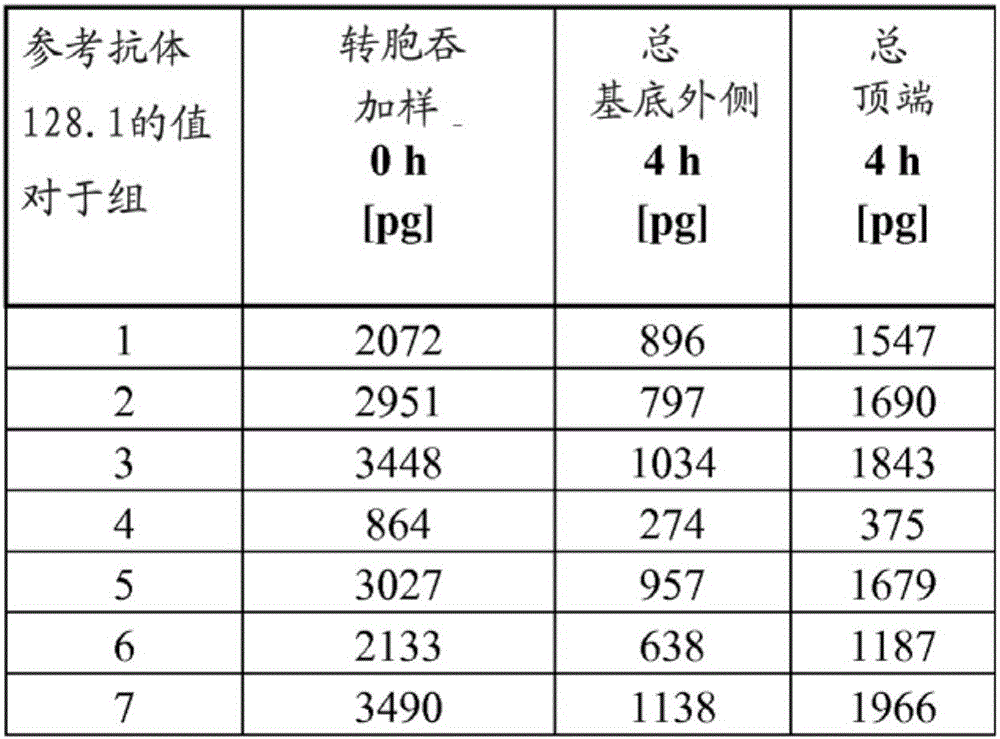
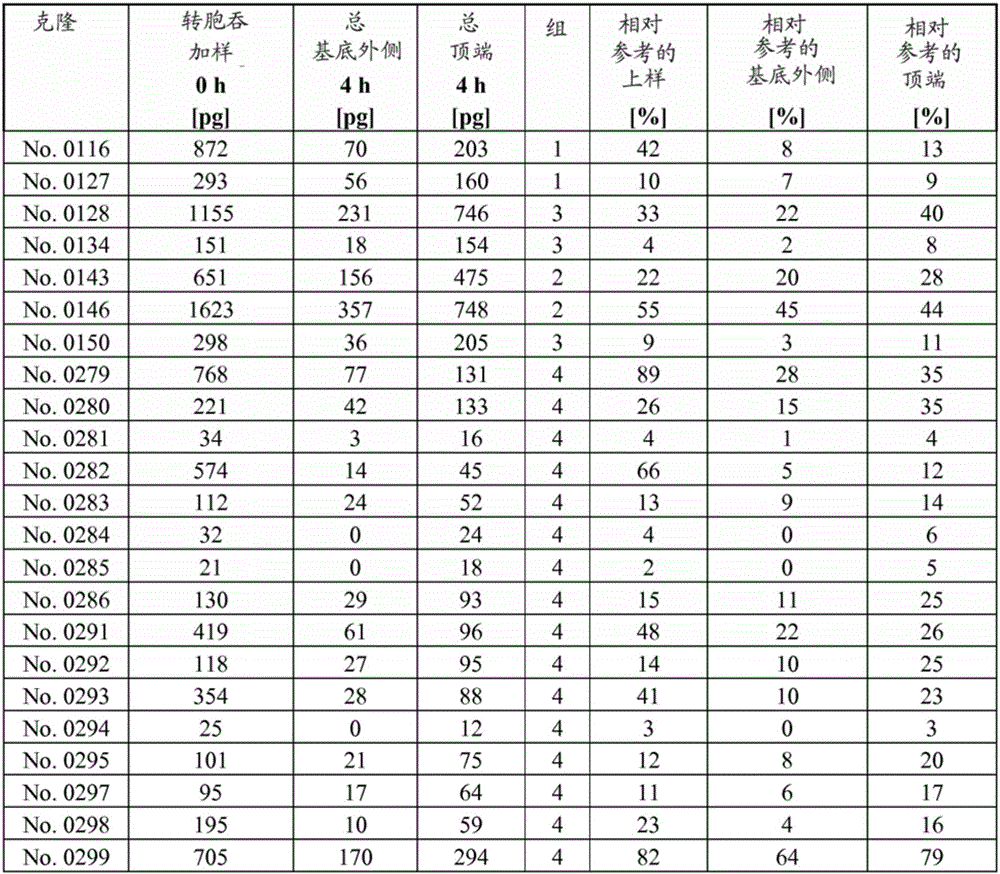
[0156] Media and supplements for hCMEC / D3 (see WO 2006 / 056879 and Weksler, B.B. et al., FASEB J.19 (2005) 1872-1874) were obtained from Lonza. hCMEC / D3 cells (23-29 passage) on collagen-coated coverslips (microscope) or in flasks to confluency.
[0157] For all transcytosis assays, high-density wells (1×10 8 hole / cm 2 ) PET membrane filter inserts (0.4 μm pore size, 12 mm diameter) are / can be used in 12-well cell culture plates. For the apical and basolateral compartments, calculate the medium volume, which is 400 µL and 1600 µL, respectively. Use / can use rat tail collagen I (7.5μg / cm 2 ) followed by coating the apical chamber of the filter insert with fibronectin (5 μg / mL), with each incubation lasting 1 h at RT. For 10-12 days in EBM2 medium, grow hCMEC / D3 cells to a confluent monolayer (~2 × 10 5 cells / cm 2 ). Air filters were / can be blocked in PBS containing 1% BSA for 1 hour or overnight (o / n) before testing and then calibrated in EBM2 for at least 1 hour.
[015...
Embodiment 1
[0568] Immunization of rabbits and mice
[0569] Immunization of mice
[0570] By transdermal administration of 100 μg carrier DNA, followed by electroporation (2 square pulses of 1000V / cm, duration 0.1ms, interval 0.125s; followed by 4 square pulses of 287.5V / cm, duration 10ms, interval 0.125s), using a plasmid expression vector encoding full-length human or macaque TfR, genetically immunized NMRI mice. Mice received 6 or 7 consecutive immunizations on days 0, 14, 28, 42, 56, 70 and 84. The fourth and sixth immunizations were performed using a vector encoding macaque TfR; a vector encoding human TfR was used for all other immunizations. Blood was drawn on days 36, 78 and 92 and serum prepared for titration by ELISA (see below). Select the animal with the highest titer for a boost on day 96: 10 iv 6 Human TF-1 cells or 50 μg recombinant human soluble TfR lacking the helical domain (human TfR extracellular domain starting at Leu122 and ending at Asn608, expressed in HEK23...
Embodiment 2
[0576] B-cell clones from rabbits
[0577] Isolation of Rabbit Peripheral Blood Mononuclear Cells (PBMC)
[0578] In total, blood samples were obtained from 6 animals (2 wild type (wt) rabbits and 4 transgenic (tg) rabbits). These rabbits were derived from 2 different rounds of immunization: the first round of immunizations used 2 wt and 2 tg rabbits, while the second round of immunizations used 2 tg rabbits (see also example "Immunization of rabbits"). Whole blood containing EDTA was diluted two-fold with 1×PBS (PAA, Pasching, Austria), followed by lymphocyte separation medium (Cedarlane Laboratories, Burlington, Ontario, Canada) according to the manufacturer's instructions. Density centrifugation. PBMCs were washed twice with 1×PBS.
[0579] EL-4 B5 medium
[0580] Supplemented with 10% FCS (Hyclone, Logan, UT, USA), 2mM glutamine, 1% penicillin / streptomycin solution (PAA, Pasching, Austria), 2mM sodium pyruvate, 10mM HEPES (PAN Biotech, Aidenbach, Germany ) and 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com