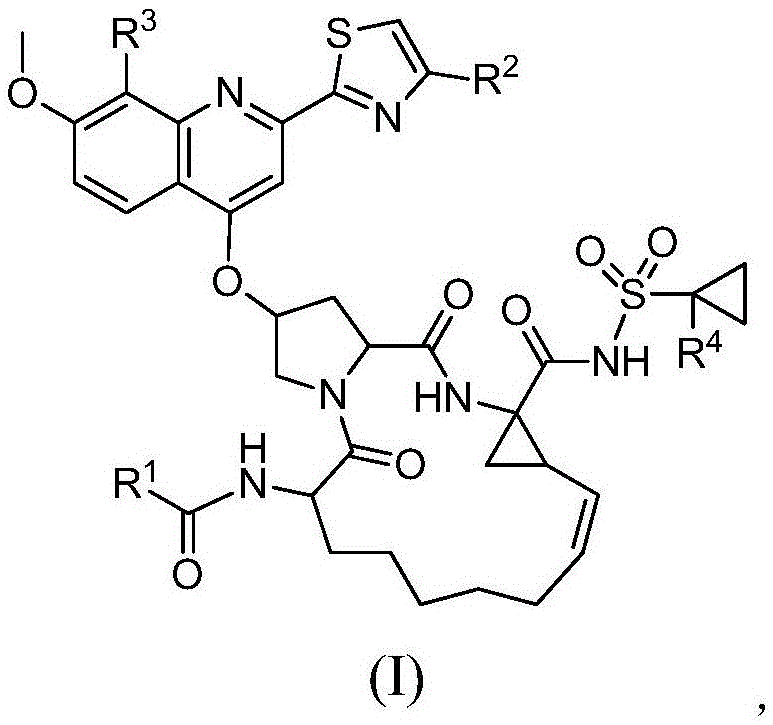
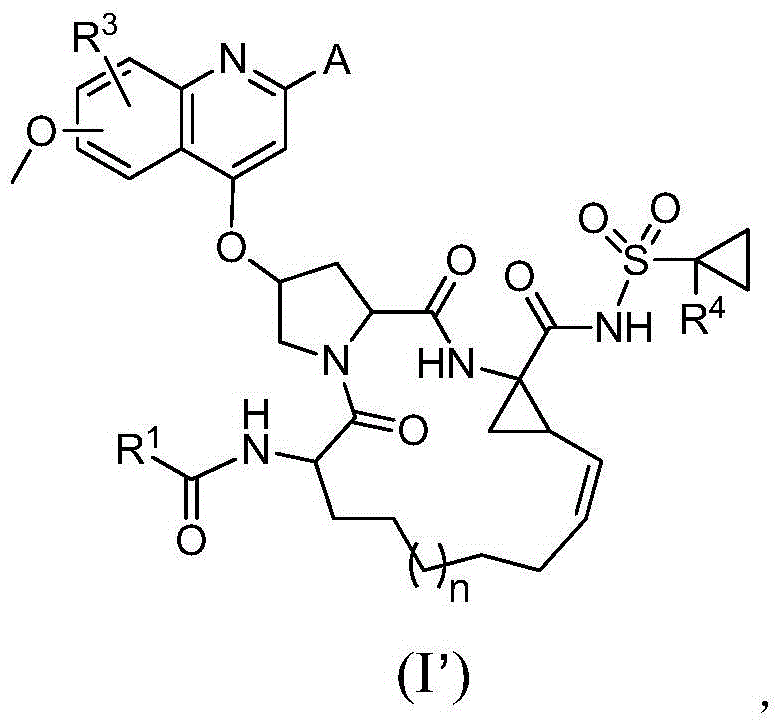
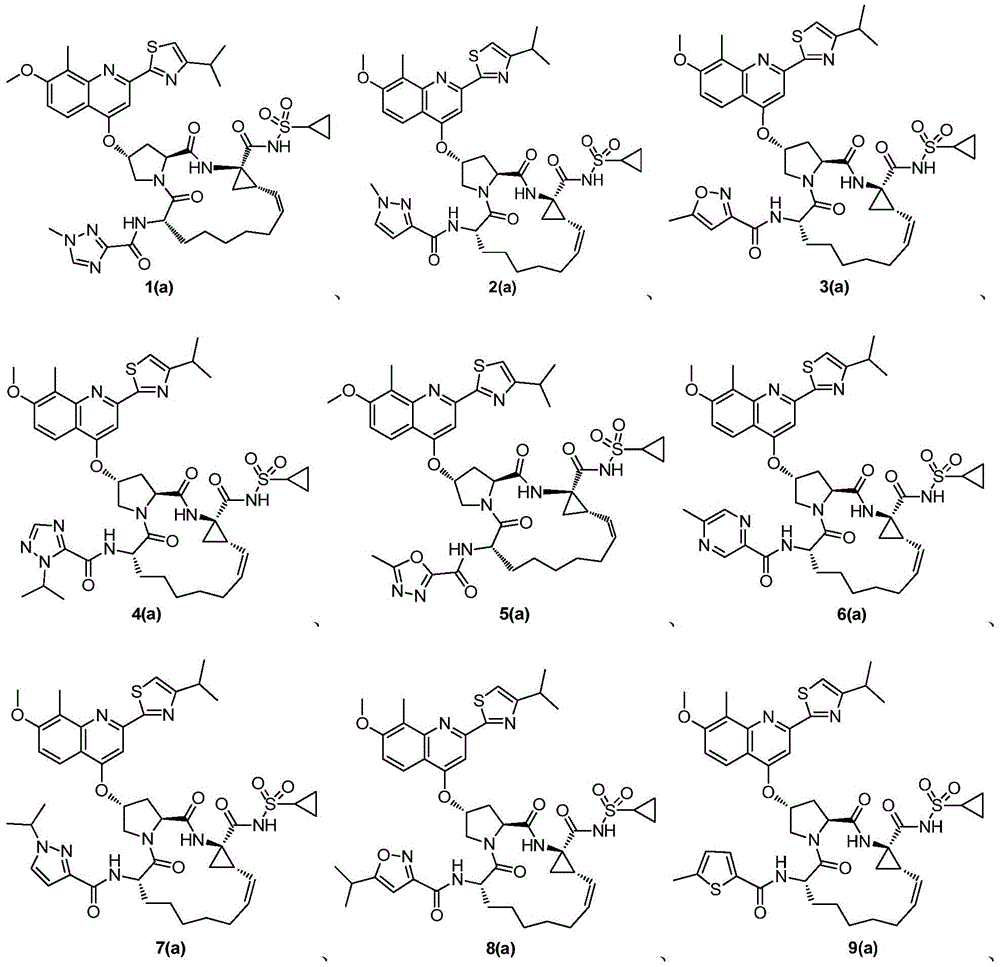
Compound adopted as hepatitis c inhibitor and application thereof in medicine
A compound and solvate technology, applied in the fields of drug combination, digestive system, organic chemistry, etc., can solve the problem of unclear clinical importance of HCV genetic heterogeneity, and achieve good inhibitory effect, good inhibitory effect, and good inhibitory activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0316]
[0317] synthetic route:
[0318]
[0319] Step 1: Synthesis of compounds 1-3
[0320] Compound 1-2 (20 g, 150 mmol) and compound 1-1 (30 g, 170 mmol) were added into ethanol (100 mL), then heated to 75° C., and stirred at this temperature for two hours. After the reaction was complete, it was lowered to room temperature, and the ethanol solution was removed under reduced pressure, 100 mL of water was added to quench the reaction, and the pH value of the mixture was adjusted to between 6 and 8 with ammonia water. Then it was extracted twice with MTBE (100mL×2), the combined organic phase was washed twice with water (50mL×2), separated, and the resulting organic phase was concentrated under reduced pressure to obtain brownish red crude product 1-3, without Further purification directly proceeds to the next reaction.
[0321] MS(ESI,pos.ion)m / z:200.3[M+1] + .
[0322] Step 2: Synthesis of compounds 1-4
[0323] Compound 1-3 (28g, 140mmol) was added to methanol...
Embodiment 2
[0334]
[0335] synthetic route
[0336]
[0337] Step 1: Synthesis of Compound 2-3
[0338] Benzaldehyde 2-1 (95.6mL, 943mmol), glycine ethyl ester hydrochloride 2-2 (131g, 943mmol) and Na 2 SO 4 (80g, 565mmol) was added into 900ml TBME, then cooled to 0°C, and triethylamine (197mL, 1414mmol) was added slowly, then warmed up to 25°C, and stirred at this temperature for 24 hours. The mixture was passed through a column with celite to remove the solid, and the organic solvent in the filtrate was removed under reduced pressure, and the solvent was removed from the obtained residue under high vacuum to obtain quantitative crude product 2-3, which was directly carried out to the next reaction without further purification.
[0339] 1 H NMR (600MHz, CDCl 3 ):δ8.28(s,1H),7.86–7.71(m,2H),7.50–7.32(m,3H),4.47–4.32(m,2H),4.29–4.18(m,2H),1.32–1.22 (m,3H)ppm.
[0340] Step 2: Synthesis of compounds 2-4
[0341] Lithium tert-butoxide (17.60g, 220mmol) was added to 250ml of tol...
Embodiment 3
[0356]
[0357] synthetic route:
[0358]
[0359] Step 1: Synthesis of compound 3-3
[0360] Compound 3-1 (100g, 460.36mmol) and compound 3-2 (100g, 564.72mmol) were dissolved in 1L of acetonitrile, then cesium carbonate (90g, 276.219mmol) and potassium carbonate (40g, 289.427mmol) were added, and the reaction The mixture was warmed to reflux for 24 hours. Cool to room temperature, filter, and remove the organic solvent from the filtrate under reduced pressure to obtain the crude product 3-3, which is directly carried out to the next reaction without further purification. MS(ESI,pos.ion)m / z:314.4[M+1] + .
[0361] Step 2: Synthesis of Compound 3-5
[0362] Compound 3-3 (150g, 478.6mmol) was dissolved in ethanol (750mL), and then a solution of KOH (81g, 1443.72mmol) in water (75mL) was slowly added dropwise. After the dropwise addition, the temperature was raised to reflux for 4 hours. After the reaction was complete, ethanol was removed under reduced pressure, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com