Anemone flaccida medicinal material HPLC-UV characteristic spectrum construction method
A technology of characteristic map and construction method, applied in the field of construction of characteristic map of traditional Chinese medicine Diwu, can solve the problems of research, lack of overall quality evaluation of Diwu medicinal materials, etc., and achieve the effect of simple operation and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
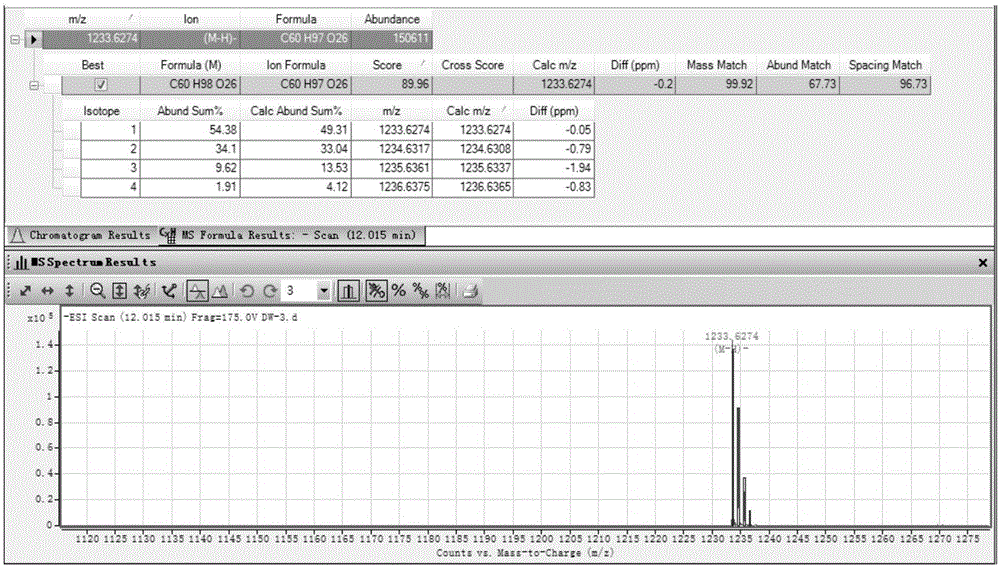
[0029] Example 1 Preparation, purification and structural identification of main chemical components in Diwu medicinal material
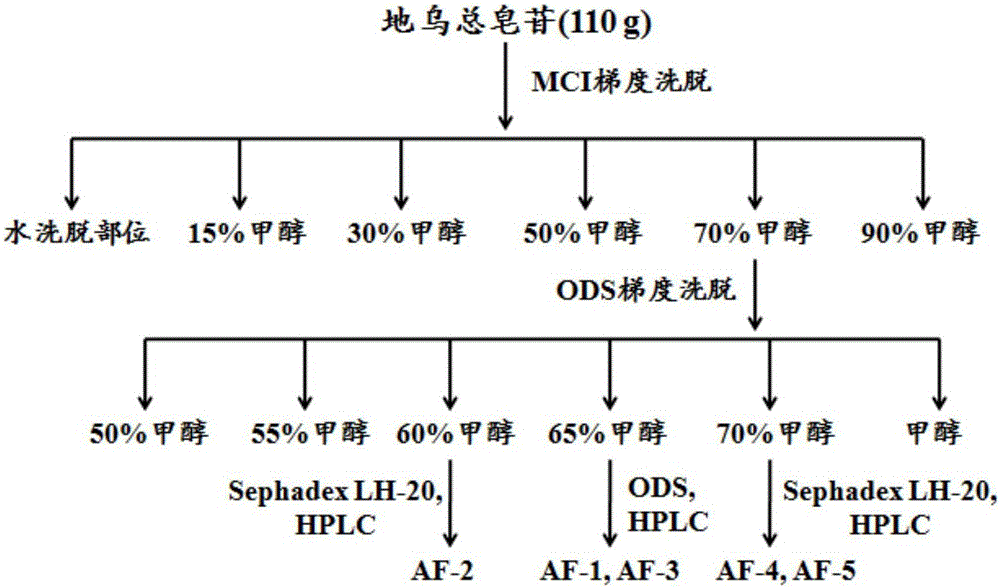
[0030] Such as figure 1 As shown, take 110g of total saponins of the root, dissolve it with 300mL of water, put the sample on the treated MCI small-pore resin column, and elute with water, 15%, 30%, 50%, 70% and 90% methanol successively, The 70% methanol eluted fraction was collected and concentrated to dryness under reduced pressure to obtain a dry extract (39.36 g). The extract was dissolved in 50% methanol, loaded on an ODS column, and eluted with a gradient of 60% to 95% methanol, and the eluted part of 60% methanol was separated by Sephadex LH-20 gel column chromatography and preparative HPLC [Cosmosil 5C 18 -MS-Ⅱ (20mm×250mm, 5μm) chromatographic column, the mobile phase is 30% acetonitrile-0.01% trifluoroacetic acid aqueous solution, the flow rate is 6.0mL min -1 ], to obtain compounds 2 (300mg) and 3 (200mg), 65% methanol elution site was...
Embodiment 2
[0071] Example 2 Construction method of characteristic map of Diwu medicinal material
[0072] (1) Preparation of reference substance solution
[0073] Accurately weigh an appropriate amount of each reference substance, add 30% acetonitrile to make anhuienoside E (0.25mg·mL -1 ), glycoside St-14a (0.25mg·mL -1 ), hemsgiganoside B (0.25mg·mL -1 ), flaccidoside II (0.5mg·mL -1 ), hederasaponin B (0.25mg·mL -1 ) of the reference substance solution, filtered, and obtained.
[0074] Table 3 Purity of reference substance
[0075]
[0076] Note: S is the reference peak
[0077] (2) preparation of need testing solution
[0078] Take about 0.5g of Diwu sample powder, weigh it accurately, put it in a stoppered Erlenmeyer flask, add 50mL of 75% methanol accurately, weigh it, extract it by ultrasonic, let it cool, make up the lost weight, shake well, and use 0.22μm Membrane filtration, take the continued filtrate, that is.
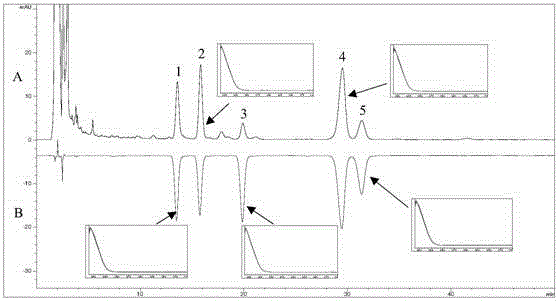
[0079] (3) Establishment of HPLC-UV chromatographic ...
Embodiment 3
[0092] Example 3 Determination Method of Characteristic Map of Diwu Medicinal Material
[0093] (1) Preparation of test solution
[0094] Take about 0.5g of Diwu medicinal material sample powder, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 50mL of 75% methanol precisely, seal it tightly, weigh it, treat it ultrasonically for 40 minutes, let it cool, weigh it again, and use Make up the lost weight with 75% methanol, shake well, filter with a 0.22μm filter membrane, and take the subsequent filtrate to obtain the test solution.
[0095] (2) Determination by high performance liquid chromatography
[0096] The need testing solution of step (1) is carried out high performance liquid chromatography to measure, and chromatographic condition is:
[0097] Agilent 1260 high performance liquid chromatography; column: Cosmosil 5C 18 -MS-Ⅱ (4.6mm×250mm, 5μm); mobile phase: acetonitrile-0.01% trifluoroacetic acid solution (30:70); detection wavelength: 205nm; flow rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com