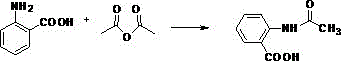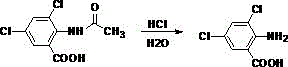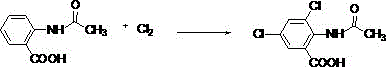Preparation method of 3,5-dichlorobenzoyl chloride
A technology of dichlorobenzoyl chloride and benzoic acid, which is applied in the preparation of carboxylic acid amides, organic compounds, carboxylate, etc., can solve the problem of low yield and achieve the effect of preventing oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 137g of anthranilic acid and 110g of acetic anhydride to a 1000mL four-necked flask, and perform the first reaction at 100°C. Stop the reaction when the impurity peak of the liquid chromatography reaches below 3% (the same below), and obtain 2-acetamidobenzene Formic acid (the required reaction time is about 12h). After the first reaction is over, add 500g of glacial acetic acid to the above-mentioned four-necked bottle, stir evenly, and then slowly introduce 150g of chlorine gas at 40°C to carry out the second reaction. Stop the reaction (the same below); the required reaction time is about 12 hours. After the second reaction, the product in the four-neck flask was taken out, filtered and washed with suction to obtain 3,5-dichloro-2-acetamidobenzoic acid as a solid. Then add 3,5-dichloro-2-acetamidobenzoic acid solid to 400g of 36.5% (mass concentration, the same below) hydrochloric acid for the third reaction, the reaction temperature is 120°C, liquid chromatogra...
Embodiment 2
[0049] Add 137 g of anthranilic acid and 110 g of acetic anhydride into a 1000 mL four-necked flask, and perform the first reaction at 120° C. to obtain 2-acetamidobenzoic acid. After the first reaction was completed, 500 g of glacial acetic acid was added into the above-mentioned four-necked flask, and after stirring evenly, 150 g of chlorine gas was slowly introduced at 40° C. to carry out the second reaction. After the second reaction, the product in the four-neck flask was taken out, suction filtered and washed to obtain a solid of 3,5-dichloro-2-acetamidobenzoic acid. Then add 3,5-dichloro-2-acetamidobenzoic acid solid into 400g of 36.5% hydrochloric acid to carry out the third reaction, the reaction temperature is 120°C, and the acetic acid generated is continuously distilled during the reaction process to obtain 3,5-dichlorobenzoic acid Chloro-2-aminobenzoic acid. Then the product of the third reaction was added to 120g of isopropanol, 400g of 36.5% hydrochloric acid, ...
Embodiment 3
[0051] Add 137 g of anthranilic acid and 120 g of acetic anhydride into a 1000 mL four-necked flask, and perform the first reaction at 120° C. to obtain 2-acetamidobenzoic acid. After the first reaction was completed, 300 g of glacial acetic acid was added into the above-mentioned four-necked flask, and after stirring evenly, 150 g of chlorine gas was slowly introduced at 40° C. to carry out the second reaction. After the second reaction, the product in the four-neck flask was taken out, suction filtered and washed to obtain a solid of 3,5-dichloro-2-acetamidobenzoic acid. Then add 3,5-dichloro-2-acetamidobenzoic acid solid into 400g of 36.5% hydrochloric acid to carry out the third reaction, the reaction temperature is 130°C, and the acetic acid produced is continuously distilled during the reaction process to obtain 3,5-dichlorobenzoic acid Chloro-2-aminobenzoic acid. Then the product of the third reaction was added to 120g of isopropanol, 400g of 36.5% hydrochloric acid, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com