A kind of fluorenone pyridine nickel nanocluster and preparation method thereof
A technology of fluorenone pyridine and nanoclusters, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of complex synthesis process, environmental pollution, high price, etc., and achieve efficient catalytic coupling reaction and electron delocalization The effect of strong resistance and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
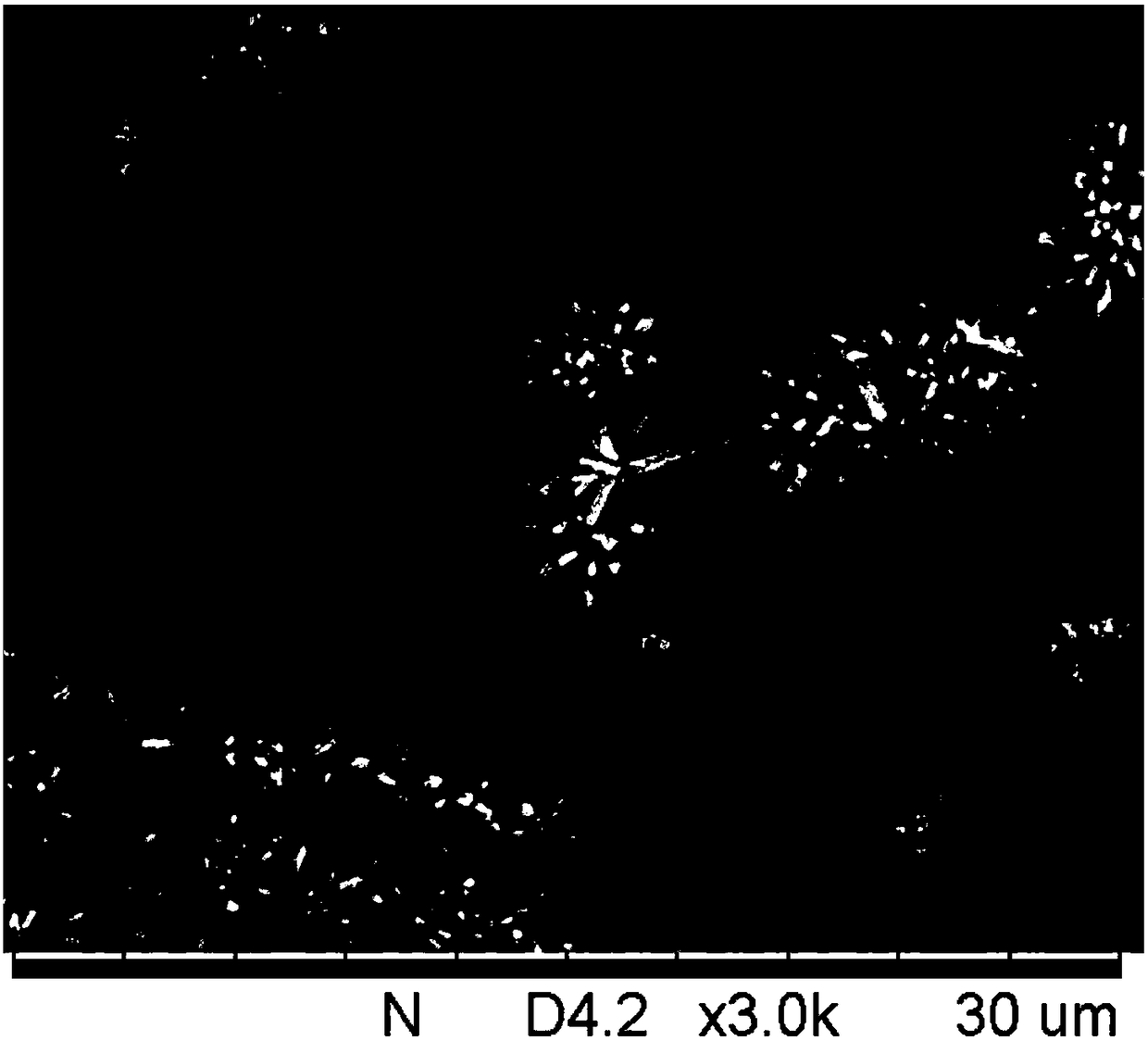
[0023] Under vigorous stirring, the isopropanol solution of fluorenone pyridine (10mmol / L, 15mL) was slowly added dropwise to nickel nitrate aqueous solution (5mmol / L, 15mL), continued vigorous stirring and reacted at 60°C for 60min, after the reaction The mixed system was ultrasonically dispersed for 30 minutes, centrifuged, and the supernatant was discarded to obtain a precipitate; the obtained precipitate was washed with deionized water and ethanol, ultrasonically dispersed, centrifuged, and the supernatant was discarded to remove free ions and unsaturated coordinated organic ligands, and then vacuum-dry the lower precipitate to obtain the fluorenone pyridine nickel nanocluster. The morphology of the nanoclusters was observed with a scanning electron microscope, as shown in figure 1 shown.
Embodiment 2
[0025] Under vigorous stirring, the propanol solution of fluorenone pyridine (20mmol / L, 15mL) was slowly added dropwise to nickel chloride aqueous solution (5mmol / L, 15mL), continued vigorous stirring and reacted at 90°C for 30min, after the reaction The mixed system was ultrasonically dispersed for 30 minutes, centrifuged, and the supernatant was discarded to obtain a precipitate; the obtained precipitate was washed with deionized water and ethanol, ultrasonically dispersed, centrifuged, and the supernatant was discarded to remove free ions and unsaturated coordinated organic ligands, and then vacuum-dry the lower precipitate to obtain the fluorenone pyridine nickel nanocluster. The morphology of the nanoclusters was observed with a scanning electron microscope.
Embodiment 3
[0027] Under vigorous stirring, the ethanol solution of fluorenone pyridine (20mmol / L, 15mL) was slowly added dropwise to nickel acetate aqueous solution (10mmol / L, 15mL), continued vigorous stirring and reacted at 90°C for 60min, and mixed The system was ultrasonically dispersed for 30 minutes, centrifuged, and the upper layer was discarded to obtain a precipitate; the obtained precipitate was washed with deionized water and ethanol, ultrasonically dispersed, and centrifuged, and the upper layer was discarded to remove free ions and uncoordinated organic ligands, and then vacuum-dry the lower precipitate to obtain the fluorenone pyridine nickel nanoclusters. The morphology of the nanoclusters was observed with a scanning electron microscope.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com