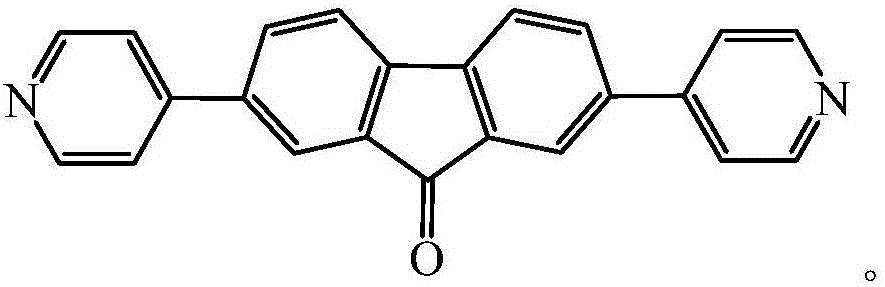
Fluorenone pyridine green fluorescent material
A technology of fluorenone pyridine and green fluorescence, which is applied in the field of fluorenone pyridine green fluorescent materials, and can solve the problems of long-wave emission of excimer associations that are easy to form, affecting the chromaticity and color stability of emitted light, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take 3.0mmol of 2,7-diiodo-9-fluorenone, 6mmol of 4-pyridineboronic acid, 30mmol of sodium carbonate, and 0.15mmol of palladium acetate in a 150mL reaction device, and add 50mL of DMF and 5mL of water; place the reaction device at 150 The reaction was carried out at ℃ for 12 h; after the reaction was completed, the liquid was separated after the reaction liquid was cooled, the aqueous phase was extracted with dichloromethane, and the organic phase was combined, and then the organic phase was washed with saturated brine for 3 times, and then dried with anhydrous sodium sulfate. Filtrate; use a rotary evaporator to remove the organic solvent in the filtrate to obtain a solid powder, then use ethyl acetate as the eluent to separate and purify by column chromatography, and finally spin dry the ethyl acetate to obtain the fluorenone pyridine green fluorescence Material.
Embodiment 2
[0020] Take 3.0mmol of 2,7-diiodo-9-fluorenone, 9mmol of 4-pyridineboronic acid, 30mmol of sodium carbonate, and 0.15mmol of palladium acetate in a 150mL reaction device, and add DMF50mL and water 5mL; put the reaction device at 90 React at ℃ for 24 h; after the reaction is completed, separate the liquid after cooling the reaction solution, extract the aqueous phase with dichloromethane, and combine the organic phase, then wash the organic phase with saturated brine for 3 times, and then dry it with anhydrous sodium sulfate. Filtrate; use a rotary evaporator to remove the organic solvent in the filtrate to obtain a solid powder, then use ethyl acetate as the eluent to separate and purify by column chromatography, and finally spin dry the ethyl acetate to obtain the fluorenone pyridine green fluorescence Material.
Embodiment 3
[0022] Take 3.0mmol of 2,7-diiodo-9-fluorenone, 7mmol of 4-pyridineboronic acid, 30mmol of sodium carbonate, and 0.15mmol of palladium acetate in a 150mL reaction device, and add DMF50mL and water 5mL; put the reaction device at 120 The reaction was carried out at ℃ for 18 h; after the reaction was completed, the liquid was separated after the reaction liquid was cooled, the aqueous phase was extracted with dichloromethane, and the organic phase was combined, and then the organic phase was washed with saturated brine for 3 times, and then dried with anhydrous sodium sulfate. Filtrate; use a rotary evaporator to remove the organic solvent in the filtrate to obtain a solid powder, then use ethyl acetate as the eluent to separate and purify by column chromatography, and finally spin dry the ethyl acetate to obtain the fluorenone pyridine green fluorescence Material.
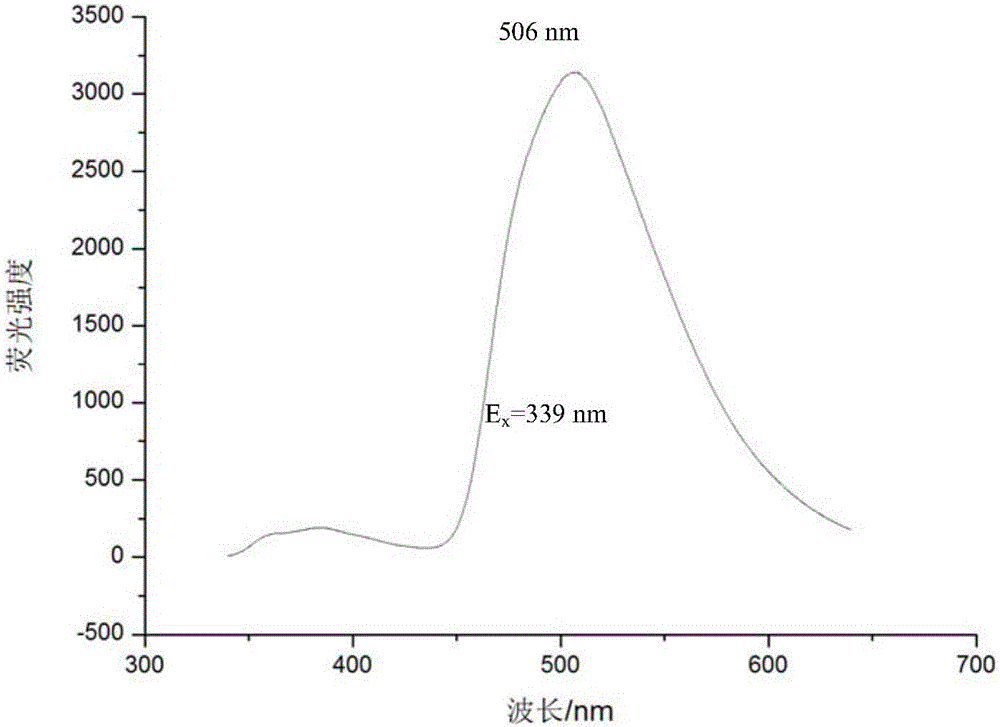
[0023] The fluorenone pyridine green fluorescent material prepared in the above examples is a powdery solid, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com