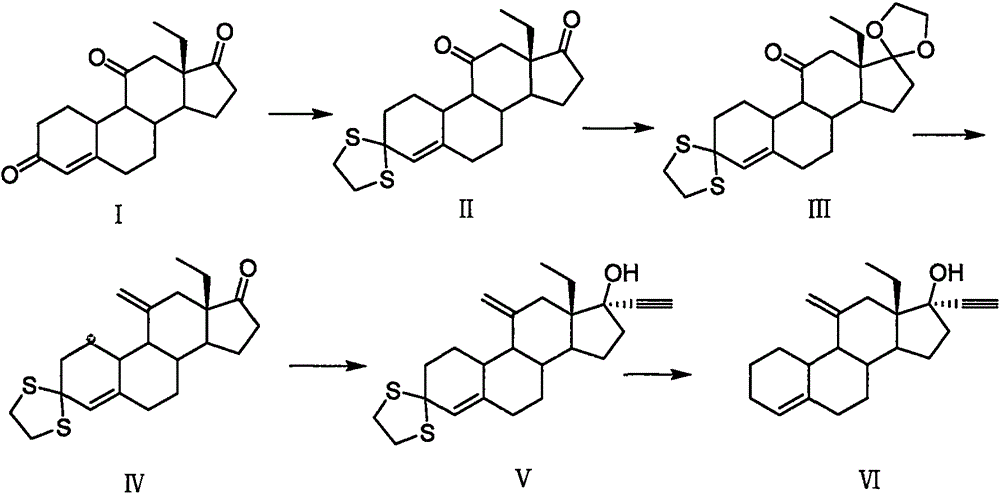
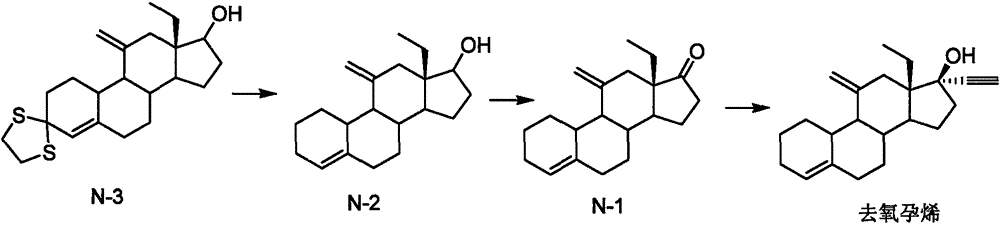
Desogestrel preparation method and midbody compound
A technology of desogestrel and steroidal compounds, applied in the direction of steroidal compounds, organic chemistry, etc., can solve the problems of high cost of process route, low purity of desogestrel, unfavorable industrial production, etc., and achieve simple and easy process, The effect of cost reduction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Compound VII
[0040] Distill 50ml of liquid ammonia into the reaction vessel, add 3g (0.43mol) of lithium metal, and react for 0.5 hours at -50-70℃. After dissolving 10g (0.026mol) of compound II in 100ml of tetrahydrofuran, drop it into the reaction In the container, react for 2h, and then slowly add 15ml of absolute ethanol dropwise. After the reaction is completed by thin layer chromatography, slowly rise to room temperature, add 50ml of purified water, precipitate a yellow solid, then filter and dry to obtain compound VII with a weight of 7.2 g, the molar yield is 94.8%.
[0041] MS(m / z): 287.4[M+H] + , 1 H-NMR(CDCl 3 ), δ5.68 (1H, t, 4-CH), δ 2.85 (2H, t, 12-CH 2 ), δ2.67(2H, t, 16-CH 2 ); 13 C-NMR, δ 215.8 (C-17), δ 210.3 (C-11), δ 137.3 (C-5), δ 122.1 (C-4).
Embodiment 2
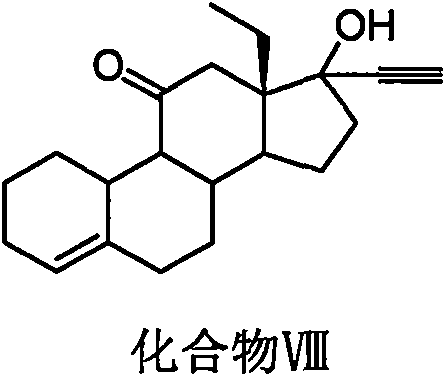
[0042] Example 2: Preparation of Compound VIII
[0043] Add 200ml (0.2mol) of n-butyllithium / n-hexane solution to 100ml of tetrahydrofuran, slowly inject acetylene gas at 15-30°C for 1 to 2 hours, and then add 10g (0.035mol) of compound VII into the reaction vessel , React at a temperature of 15-30 ℃ for 0.5 to 1 hour, after the completion of the reaction detected by thin layer chromatography, add 20ml saturated ammonium chloride solution and 50ml dichloromethane, extract the organic phase, dry over anhydrous magnesium sulfate, and then reduce pressure Concentrated to obtain compound VIII with a weight of 9.5 g and a molar yield of 87.0%.
[0044] MS(m / z): 314.4[M+H] + , 1 H-NMR(CDCl 3 ), δ 5.67 (1H, t, 4-CH), δ 5.35 (1H, s, 17-OH), δ 3.09 (1H, s, 17-OH), δ 2.89 (2H, t, 12 -CH 2 ); 13 C-NMR, δ219.7(C-11), δ137.7(C-5), δ122.2(C-4), δ84.1(C-20), δ81.1(C-17), δ73 .6(C-22).
Embodiment 3
[0045] Example 3: Preparation of Compound VIII
[0046] 14g (0.125mol) potassium tert-butoxide was added to 100ml of ether, and acetylene gas was slowly introduced at a temperature of 15-30°C for 1 to 2 hours, and then 10g (0.035mol) of compound VII was added to the reaction vessel. React at 30°C for 0.5 to 1 hour. After TLC detects the completion of the reaction, add 20ml of saturated ammonium chloride solution and 50ml of dichloromethane, extract the organic phase, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure to obtain compound VIII The weight is 9.1g, and the molar yield is 83.3%.
[0047] MS(m / z): 314.4[M+H] + , 1 H-NMR(CDCl 3 ), δ 5.67 (1H, t, 4-CH), δ 5.35 (1H, s, 17-OH), δ 3.09 (1H, s, 17-OH), δ 2.89 (2H, t, 12 -CH 2 ); 13 C-NMR, δ219.7(C-11), δ137.7(C-5), δ122.2(C-4), δ84.1(C-20), δ81.1(C-17), δ73 .6(C-22).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com