Eugenol epoxy resin and preparing method and application thereof
A technology of eugenol epoxy resin and eugenol, which is applied in the direction of epoxy resin glue, epoxy resin coating, adhesive type, etc., can solve the problems of strong dependence on petroleum resources, strong biological toxicity, etc., and achieve high vitrification Transformation temperature and mechanical strength, no biotoxicity, effect of reducing dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
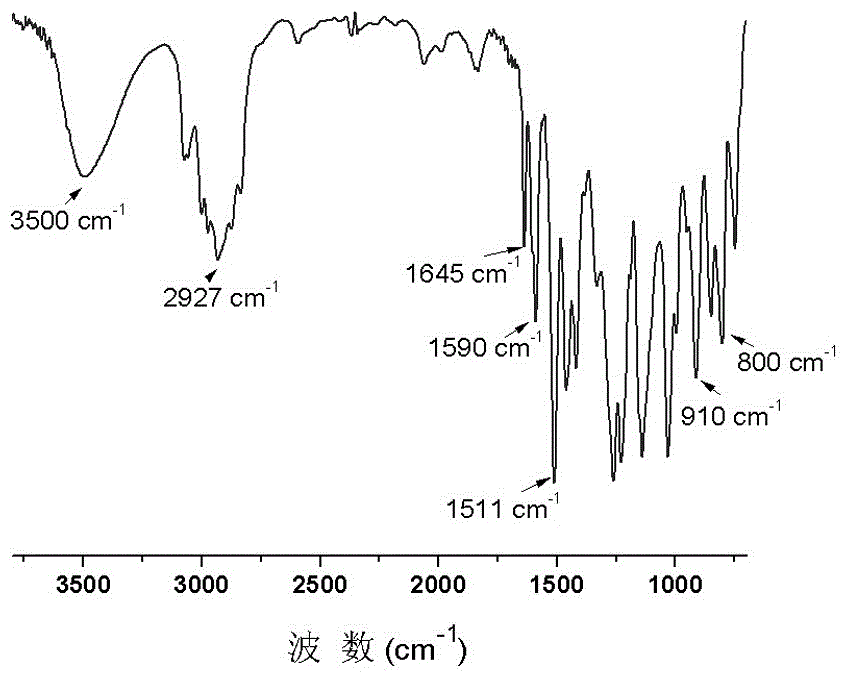
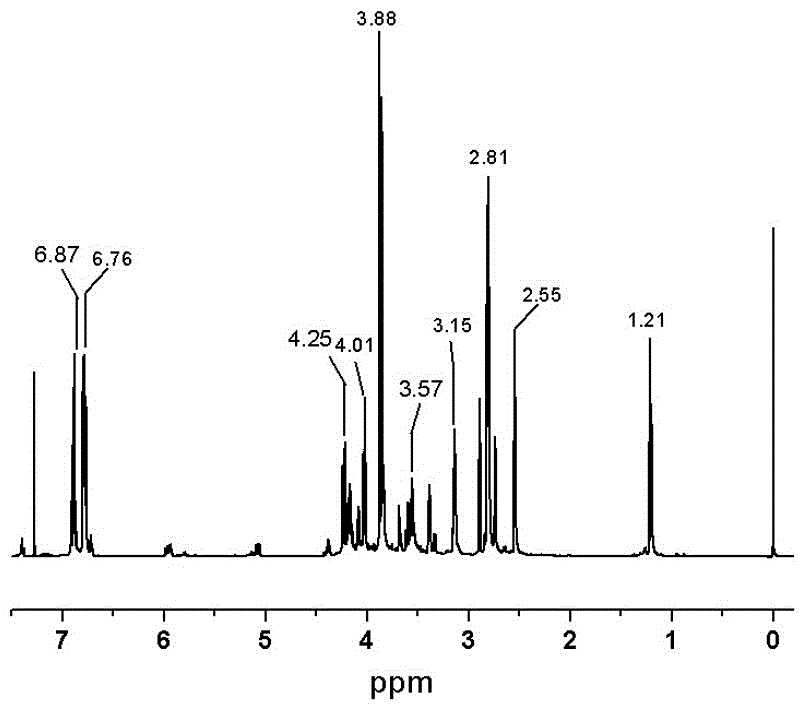
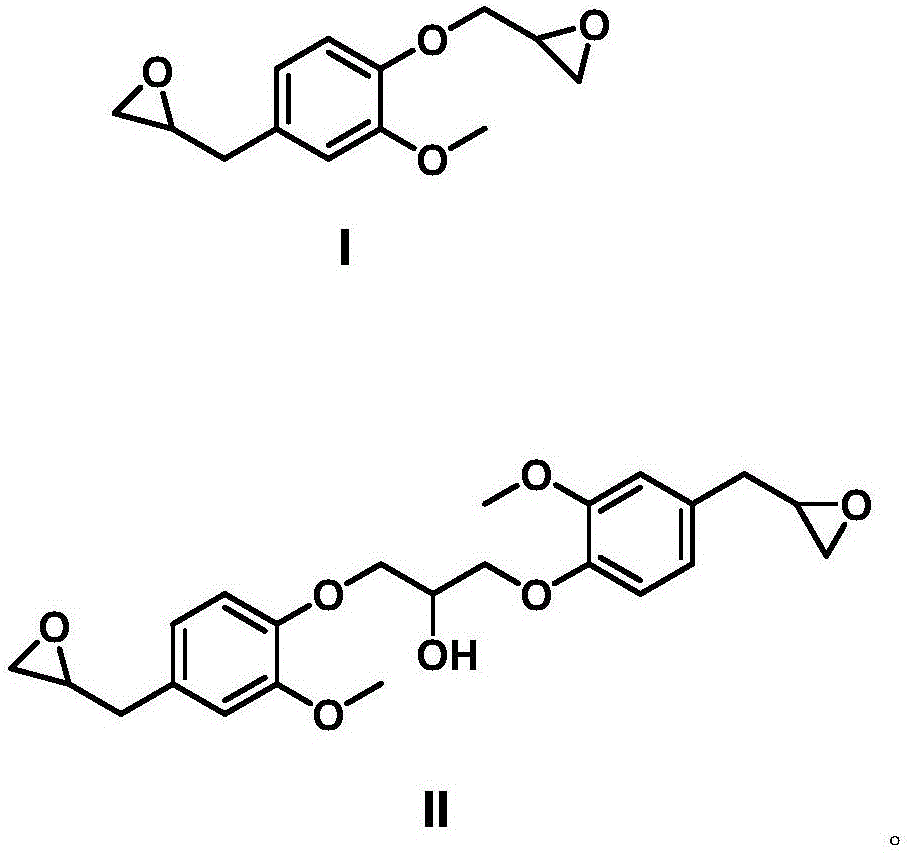
[0038] Condensation: Add 100g eugenol and 250g epichlorohydrin into a four-necked flask equipped with a thermometer, agitator and condenser, and add 1.5g hexadecyltrimethylammonium bromide, mix and stir, and heat up under nitrogen protection to 120°C, react for 3h; then cool the reaction system to below 80°C, add 88.2mL saturated NaOH solution to close the ring, control the temperature at 70-80°C, react for 2h; extract the obtained reaction solution twice with brine, dry, The excess epichlorohydrin was removed under reduced pressure to obtain an intermediate product, which was a light yellow transparent liquid at room temperature.
[0039] Oxidation: Dissolve the intermediate product in dichloromethane, add 129g m-chloroperoxybenzoic acid, and stir at 25°C for 48 hours; the obtained reaction solution is filtered to remove m-chloroperoxybenzoic acid, and then extracted with sodium bisulfite solution to starch potassium iodide The test paper does not turn blue, and then extracte...
Embodiment 2
[0045]Condensation: Add 100g eugenol, 35.2g sodium hydroxide and 300g epichlorohydrin into a four-necked flask with a thermometer, agitator and condenser, and add 1.5g hexadecyltrimethylammonium bromide, mix Stir, raise the temperature to 100°C under the protection of nitrogen, and react for 4 hours; the obtained reaction solution is extracted twice with brine, dried, and excess epichlorohydrin is removed under reduced pressure to obtain an intermediate product, which is a light yellow transparent liquid at room temperature.
[0046] Oxidation: Dissolve the intermediate product in dichloromethane, add 129g m-chloroperoxybenzoic acid, and stir at 0°C for 48 hours; the obtained reaction solution is filtered to remove m-chloroperoxybenzoic acid, and then extracted with sodium bisulfite solution to starch potassium iodide The test paper does not turn blue, then extracts with sodium carbonate solution, dries, and desolventizes under reduced pressure to obtain eugenol epoxy resin, wh...
Embodiment 3
[0048] Condensation: Add 100g eugenol and 270g epichlorohydrin into a four-necked flask equipped with a thermometer, agitator and condenser, and add 1.5g hexadecyltrimethylammonium bromide, mix and stir, and heat up under nitrogen protection to 120°C, react for 3h; then cool the reaction system to below 80°C, add 88.2mL saturated NaOH solution to close the ring, control the temperature at 70-80°C, and react for 2h; extract the reaction solution twice with brine, dry, reduce The excess epichlorohydrin was removed by pressure to obtain the intermediate product, which was a light yellow transparent liquid at room temperature.
[0049] Oxidation: Dissolve the intermediate product in dichloromethane, add 129g m-chloroperoxybenzoic acid, and stir at 0°C for 48 hours; the obtained reaction solution is filtered to remove m-chloroperoxybenzoic acid, and then extracted with sodium bisulfite solution to starch potassium iodide The test paper does not turn blue, and is extracted with sodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com