Method for degrading casein by utilizing recombinant bacillus subtilis proline aminopeptidase
A technology of proline aminopeptidase and leucine aminopeptidase, which is applied in the field of casein degradation, can solve the problems of inability to cut polypeptides, achieve the effects of improving the depth of hydrolysis, good application prospects, and simplifying the extraction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
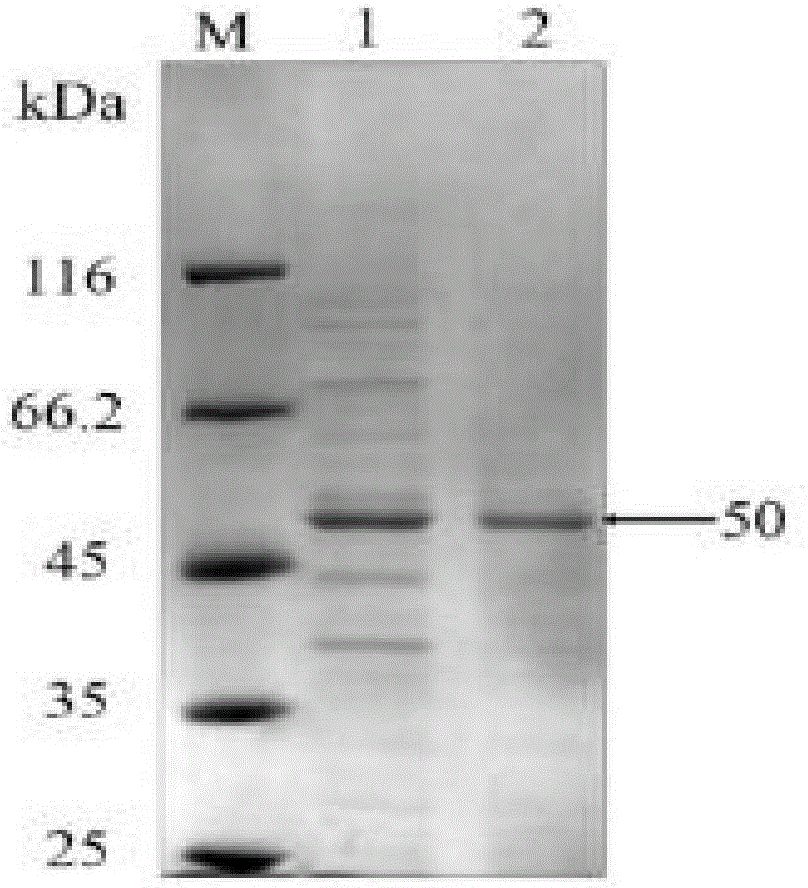
[0035] Embodiment 1: Preparation of recombinant subtilis proline aminopeptidase
[0036] Take 40mL of recombinant Bacillus subtilis WB600 fermentation broth cultured for 24h, centrifuge at 10,000rpm and 4°C for 10min, take the supernatant, discard the precipitate, and obtain the crude enzyme solution, which is then concentrated by ultrafiltration, using ultrafiltration with a molecular weight cut-off of 30kDa Centrifuge tube, the ultrafiltration condition is 5000rpm, centrifuge at 4°C for 20min, the concentration factor is controlled at 3-4 times, the ultrafiltration concentrated solution is dialyzed in 1L buffer A at 4°C overnight, and 5mL of the dialyzate is used for nickel affinity chromatography Column, the flow rate is maintained at 1mL / min, first eluted with buffer A of 5 column volumes, and then eluted with buffer B of 5 column volumes, according to the changes in the readings of the UV detector, the eluate is collected in batches, and the obtained The eluate was dialyz...
Embodiment 2
[0037] Embodiment 2: Recombinant subtilis proline aminopeptidase small molecule substrate selectivity
[0038] Prepare 1mM synthetic dipeptides and peptides with 0.05M PBS pH 7.5 buffer: Pro-Leu, Pro-Lys, Pro-Pro, Leu-Pro, Pro-Phe-Gly-Lys, Pro-Leu-Ser-Arg- Thr-Leu-Ser-Val-Ala-Ala-Lys-Lys.
[0039] Take 0.9 mL of dipeptide and polypeptide solutions respectively, and then add 0.1 mL of enzyme solution diluted with 0.05 M PBS pH 7.5 buffer solution (the enzyme solution is obtained by the method described in Example 1, and the enzyme activity is 15.4 U / mL).
[0040]After reacting at 50°C for 10 minutes, immediately add 2.5mL acetic acid and 2.5mL ninhydrin solution, bathe in boiling water for 30min, measure the absorbance at 480nm, the blank control is 0.1mL 0.05M PBS pH 7.5 buffer solution instead of the diluted enzyme solution, to The highest hydrolysis ability is 100%. The results showed that the relative activity of hydrolyzing Pro-Leu was 69.5%, the relative activity of Pro...
Embodiment 3
[0040]After reacting at 50°C for 10 minutes, immediately add 2.5mL acetic acid and 2.5mL ninhydrin solution, bathe in boiling water for 30min, measure the absorbance at 480nm, the blank control is 0.1mL 0.05M PBS pH 7.5 buffer solution instead of the diluted enzyme solution, to The highest hydrolysis ability is 100%. The results showed that the relative activity of hydrolyzing Pro-Leu was 69.5%, the relative activity of Pro-Lys was 74.4%, the relative activity of Pro-Pro was 44.1%, the relative activity of Leu-Pro was 0, and the relative activity of Pro-Phe- The relative activity to Gly-Lys was 100%, and the relative activity to Pro-Leu-Ser-Arg-Thr-Leu-Ser-Val-Ala-Ala-Lys-Lys was 17.4%. The ability of recombinant proline aminopeptidase to hydrolyze different small molecular substrates is different, and it is selective for the hydrolysis of small molecular substrates. Embodiment 3: the effect of recombinant subtilis proline aminopeptidase in casein degradation
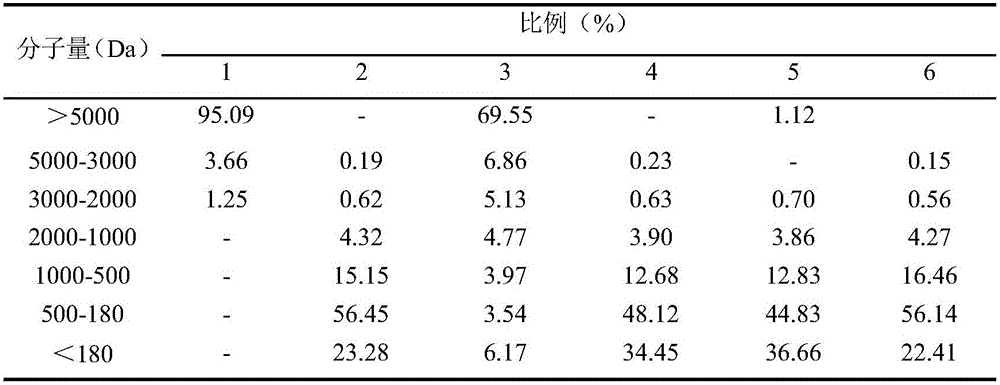
[0041] Prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com