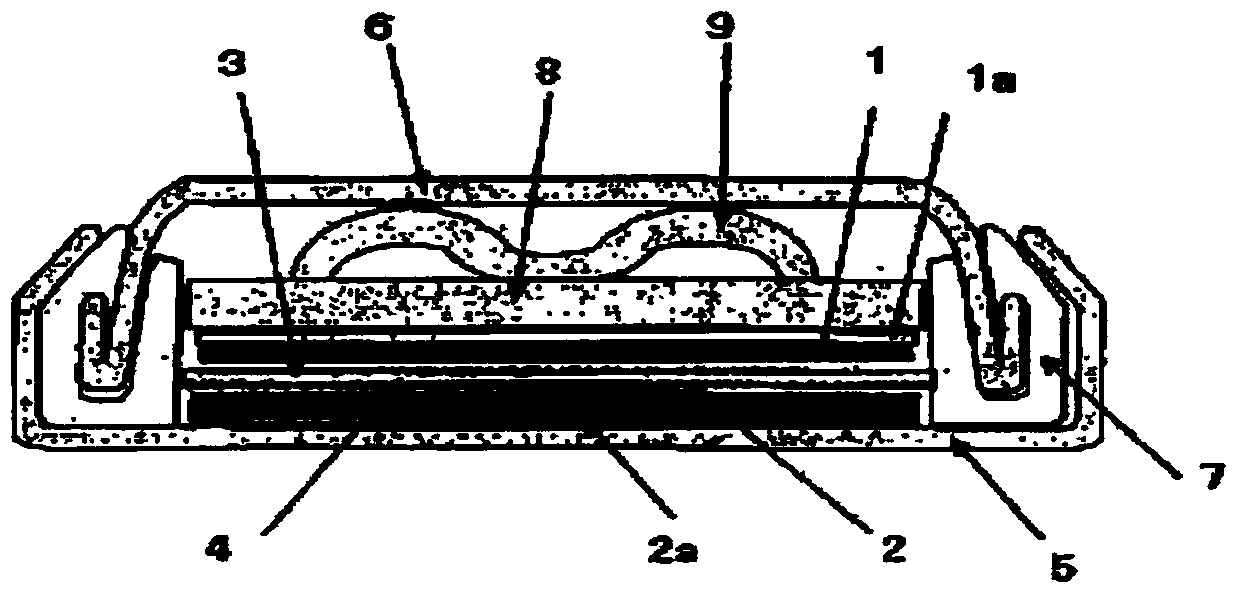
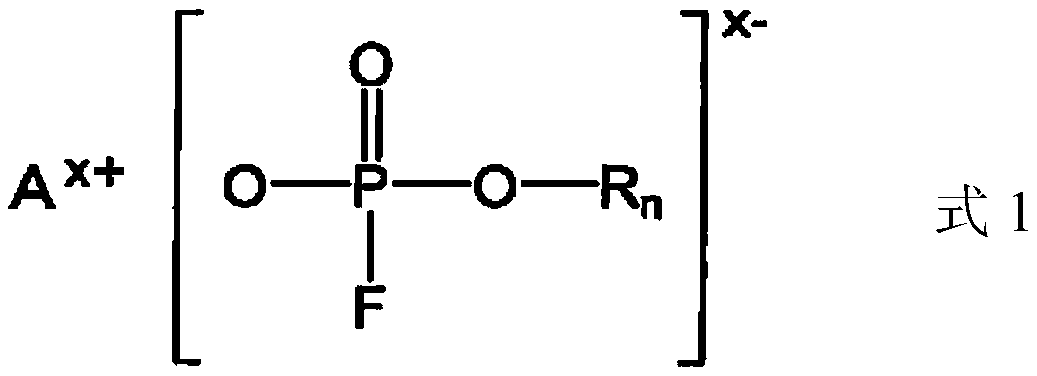
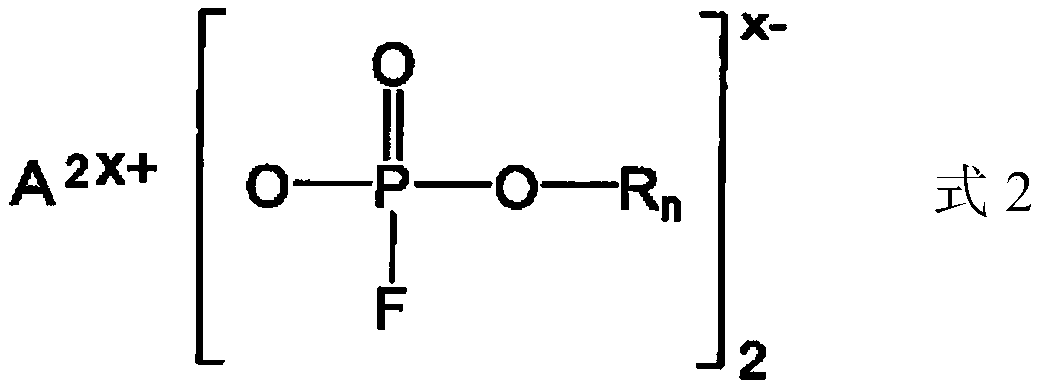
Nonaqueous electrolytic solution containing monofluorophosphate salt and nonaqueous electrolytic solution battery using same
A technology of non-aqueous electrolyte and monofluorophosphate, which is applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolyte batteries, organic electrolyte batteries, etc., can solve the problem of low solubility and achieve the effect of inhibiting decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0084] [Production Example 1] Production of lithium methyl monofluorophosphate
[0085] 7.6 g (0.18 mol) of lithium chloride, 25.0 g (0.16 mol) of phosphorus oxychloride, and 62.5 g of dimethyl carbonate were weighed into a 250 mL container made of PFA. Next, 2.9 g (0.16 mol) of pure water was dripped over 30 minutes, stirring at 10 degreeC under nitrogen sealing. After stirring for 30 minutes, 5.2 g (0.16 mol) of anhydrous methanol (water content 0.1%) was slowly added dropwise over 30 minutes while stirring. Furthermore, after stirring for 30 minutes, 34.3 g (0.24 mol in conversion of hydrogen fluoride) of 14% hydrogen fluoride dimethyl carbonate solution was added, and it stirred at 10 degreeC for 30 minutes. Thereafter, stirring was carried out at 120° C. for 1 hour while sealing with nitrogen. After heating at 120°C for 2 hours, the remaining solvent and reaction by-products were distilled off. Then, it was cooled to room temperature to obtain crude lithium methyl mono...
manufacture example 2
[0086] [Production Example 2] Production of lithium ethyl monofluorophosphate
[0087] 7.6 g (0.18 mol) of lithium chloride, 25.0 g (0.16 mol) of phosphorus oxychloride, and 62.5 g of diethyl carbonate were weighed into a 250 mL container made of PFA. Next, 2.9 g (0.16 mol) of pure water was dripped over 30 minutes, stirring at 10 degreeC under nitrogen sealing. After stirring for 30 minutes, 7.4 g (0.16 mol) of absolute ethanol (water content 0.5%) was slowly added dropwise while stirring for 30 minutes. Furthermore, after stirring for 30 minutes, 34.3 g (0.24 mol in conversion of hydrogen fluoride) of 14% hydrogen fluoride dimethyl carbonate solution was added, and it stirred at 10 degreeC for 30 minutes. Thereafter, stirring was carried out at 120° C. for 1 hour while sealing with nitrogen. After heating at 120°C for 2 hours, the remaining solvent and reaction by-products were distilled off. Then, it was cooled to room temperature to obtain crude ethyl monofluorophosphat...
manufacture example 3
[0088] [Production Example 3] Production of lithium hexyl monofluorophosphate
[0089] 7.6 g (0.18 mol) of lithium chloride, 25.0 g (0.16 mol) of phosphorus oxychloride, and 62.5 g of dimethyl carbonate were weighed into a 250 mL container made of PFA. Next, 2.9 g (0.16 mol) of pure water was dripped over 30 minutes, stirring at 10 degreeC under nitrogen sealing. After stirring for 30 minutes, 16.3 g (0.16 mol) of anhydrous n-hexanol was slowly added dropwise while stirring for 30 minutes. Furthermore, after stirring for 30 minutes, 34.3 g (0.24 mol in conversion of hydrogen fluoride) of 14% hydrogen fluoride dimethyl carbonate solution was added, and it stirred at 10 degreeC for 30 minutes. Then, it heated at 120 degreeC for 2 hours, and the remaining solvent and reaction by-products were distilled off. Then, it was cooled to room temperature to obtain crude lithium hexyl monofluorophosphate. As a result of purification treatment of the obtained crude product, a peak of li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com