Biological materials and therapeutic uses thereof
A use, biologically active technology in the field of biological materials and their therapeutic uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] Example 1: General method
[0303] Reagent
[0304] Zymosan, methylated BSA and Freund's complete adjuvant (Freund's complete adjuvant), anti-FLAG M2 antibody (mouse monoclonal antibody), blasticidin, and isotype control antibody (mouse IgG2a , IgG1) were from Sigma-Aldrich (Dorset, UK). Hypnorm (fentanyl-fluanone) was from VetaPharma Ltd (Leeds, UK). Limulus amebocyte lysate assay was from Associates of Cape Cod (Liverpool, UK). Wild-type human embryonic kidney (HEK293-EBNA) cells were from Invitrogen (Groningen, Netherlands). M-CSF and murine IL-1β were from PeproTech (Neuilly-Sur-Seine, France). DMEM, RPMI 1640, fetal bovine serum (FBS), penicillin / streptomycin, antibiotic-antimycotic solution PSA, and β-mercaptoethanol were from PAA Laboratories (Yeovil, UK) . HEK293 cell lines stably expressing human TLR2 and TLR4 / CD14 / MD-2, polymyxin B, msbB LPS, and functionally blocked TLR2 (clone: TL2.1; isotype: mouse IgG2a) and TLR4 antibody (clone : HTA125; isotype:...
Embodiment 2
[0319] Embodiment 2: the synthesis of recombinant protein
[0320] Proteins corresponding to each domain of tenascin-C (TA, EGF-L, various TNIII repeats, and FBG) were synthesized and purified. The recombinant protein synthesized is depicted in Figure 9 middle.
[0321] Reagent
[0322] Pfu Turbo polymerase was from Stratagene (Amsterdam, Netherlands). Easy mix 50 PCR tubes were from Molecular Bioproducts (Lutterworth, UK). RNeasy kit and Ni 2+ -NTA-agarose columns were from Qiagen (Crawley, UK). The pCR Blunt vector, pCEP4 plasmid vector, human embryonic kidney (HEK293-EBNA) cells and 4%-12% Bis-Tris gradient gel were from Invitrogen (Groningen, Netherlands). The pET32b vector and BL21(DE3) Rosetta cells were from Novagen (Kent, UK). HiTrapQ column, HiTrap S column, Sephacryl S500 HR column and heparin sepharose column were from Amersham (Buckinghamshire, UK).
[0323] Restriction enzymes were obtained from New England BioLabs (Hitchin, UK). DMEM, fetal bovine serum...
Embodiment 3
[0348] Embodiment 3: animal model
[0349] Zymosan-induced arthritis
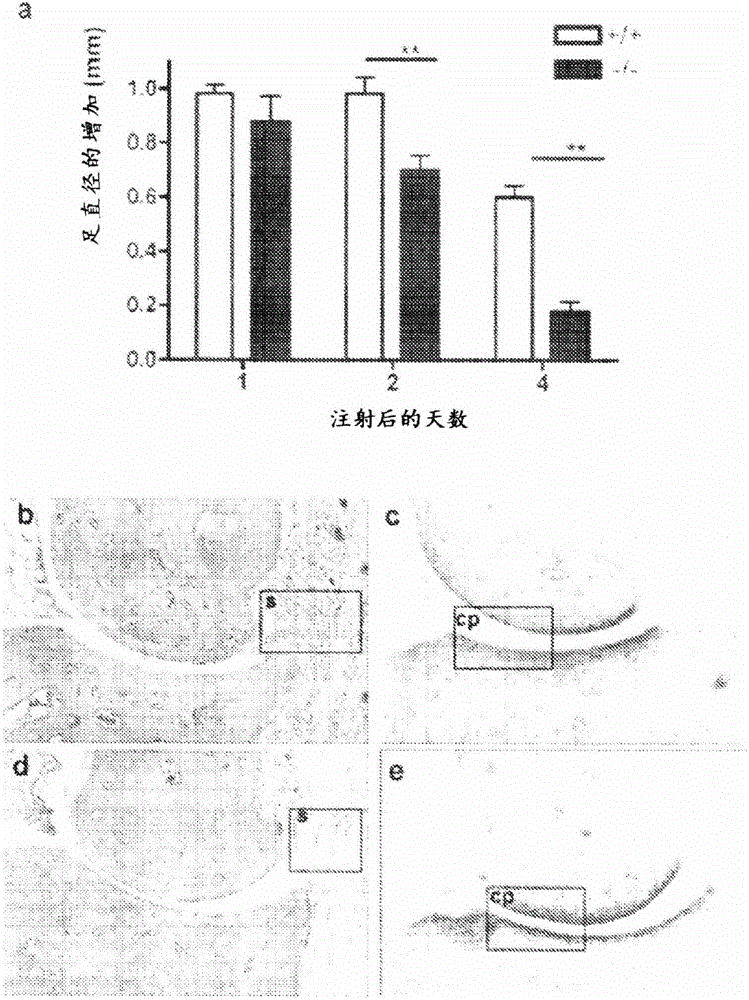
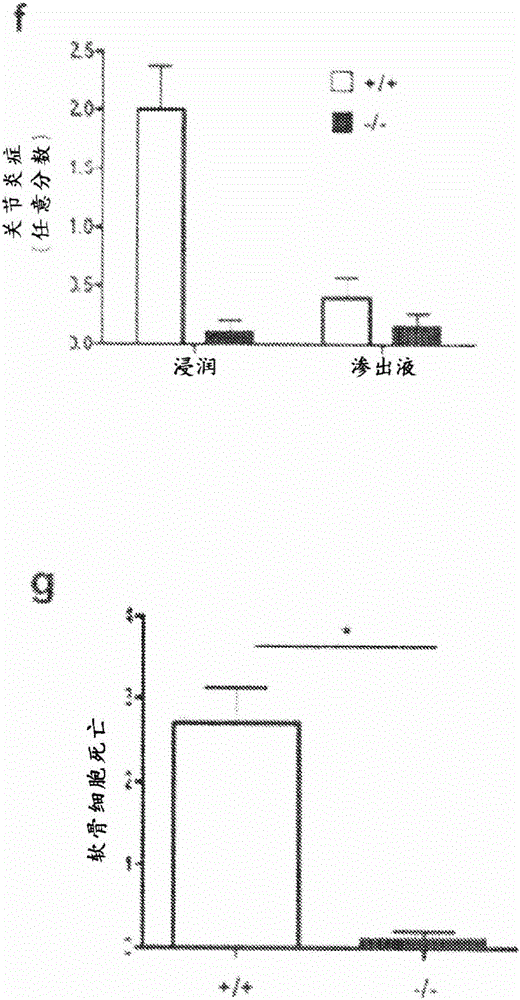
[0350] Zymosan-induced arthritis (ZIA) was induced in tenascin-C deficient and wild-type mice by injection of zymosan (Saccharomyces cerevisiae) as described in Keystone (1977). Prepare zymosan by dissolving 15 mg of zymosan in 1 ml of sterile PBS. The solution was boiled twice and sonicated. Mice were anesthetized by intraperitoneal injection of 150 μl of Hypnorm (diluted 1:10 in sterile water), followed by injection of zymosan (10 μl) into the right footpad (d=0).
[0351] Control mice received an injection of 10 μl of PBS alone or no injection. For the gross assessment of arthritis, the thickness of each hind paw was measured daily with a micrometer (Kroeplin, Schluchlem, Germany) and the diameter was expressed as the average of each inflamed hind paw per mouse. value.
[0352] After completion of the experiment (days = 4), mice were euthanized and hind paws were fixed in 10% (v / v) buffered formalin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com