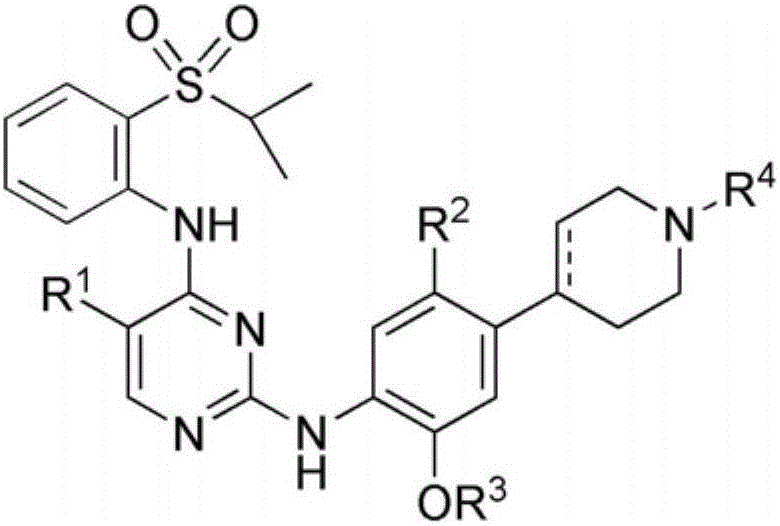
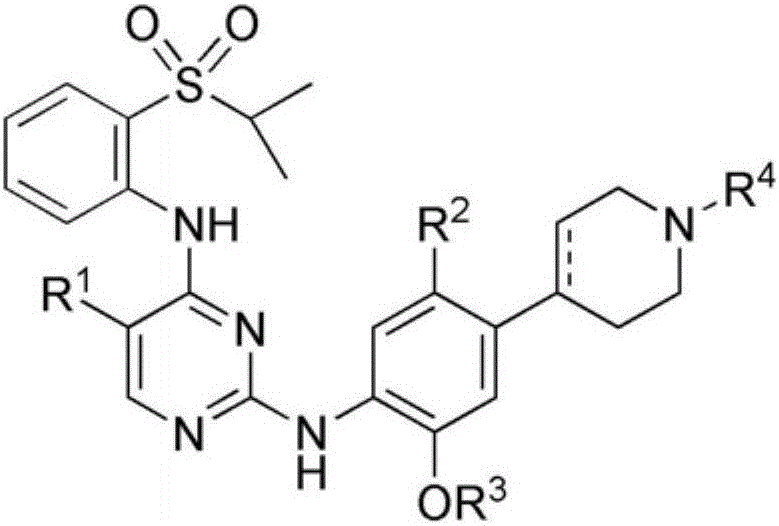
Pyrimidine-2,4-diamine derivative and pharmaceutical anticancer composition containing same as active ingredient
A kind of technology of diamine derivative and pyrimidine, which is applied in the field of pharmaceutical compositions for preventing or treating cancer, and achieves the effect of improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
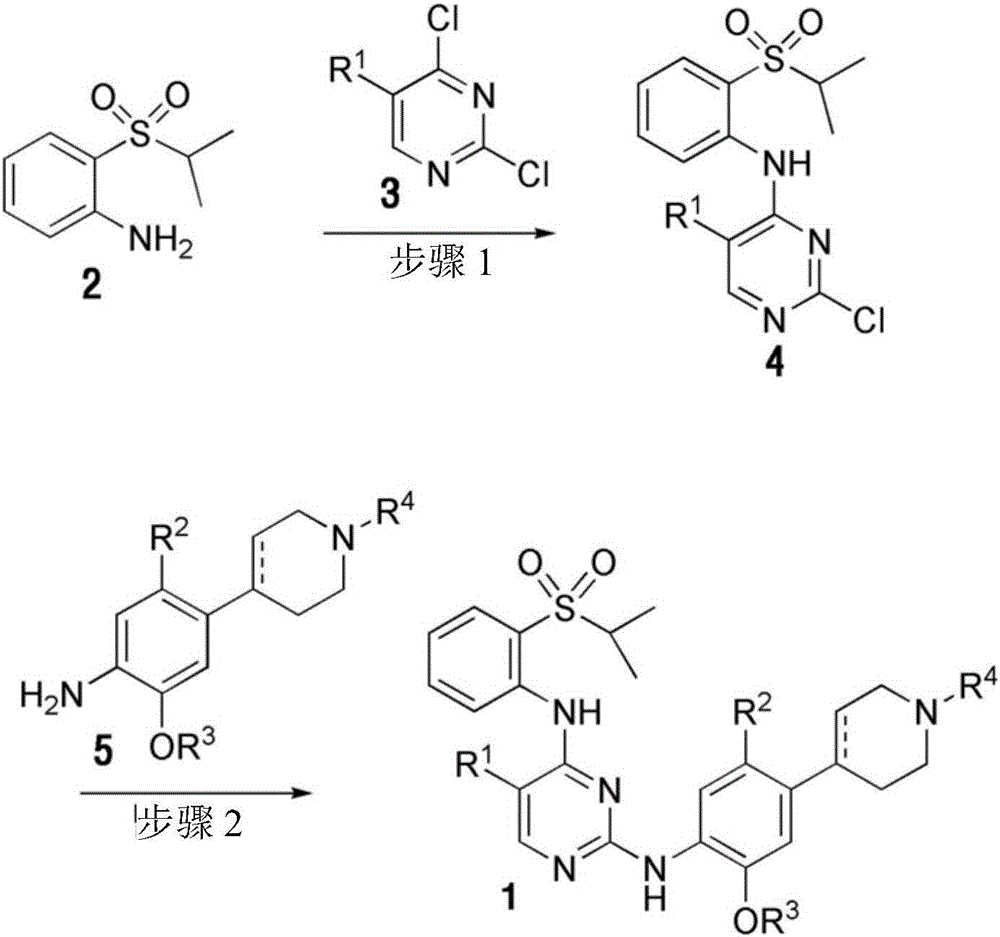
[0106] In the preparation method of the present invention, step 1 is to obtain the compound represented by formula 4 by reacting the compound represented by formula 2 with the compound represented by formula 3.
[0107] Specifically, the compound represented by Formula 4 is prepared by reacting the compound represented by Formula 2 with the compound represented by Formula 3 via alkylation in the presence of an organic solvent and a base.
[0108] At this time, the organic solvent used herein is selected from the group consisting of tetrahydrofuran; dioxane; ether solvents including diethyl ether and 1,2-dimethoxyethane; lower alcohols including methanol, ethanol , propanol and butanol; dimethylformamide (DMF), dimethylsulfoxide (DMSO), Tosylate, Chlorobenzenesulfonate, Xylenesulfonate, Phenylacetate, Phenylpropionate, Phenylbutyrate, Citrate, Lactate, Beta-Hydroxybutyrate, Glycolate, malate, tartrate, methanesulfonate, propanesulfonate, naphthalene-1-sulfonate, naphthalene-2...
Embodiment 1
[0129] Example 1: 5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-methoxy-4-(1,2,3,6-tetrahydropyridine-4- Preparation of yl)phenyl)pyrimidine-2,4-diamine
[0130] Step 1: 4-(4-((5-Chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-3-methoxyphenyl)- Preparation of tert-butyl 5,6-dihydropyridine-1(2H)-carboxylate
[0131]
[0132] 2,5-dichloro-N-(2-(isopropylsulfonyl)phenyl)pyrimidin-4-amine (1.6g, 4.6mmol) was dissolved in 25ml of THF, and 4-(4- Amino-3-methoxyphenyl)-5,6-dihydropyridine-1(2H)-carboxylic acid tert-butyl ester (1.4 g, 4.6 mmol). Add 4,5-bisdiphenylphosphine-9,9-dimethylxanthene to it in sequence (266mg, 0.46mmol) and Cs 2 CO 3 (4.5 g, 13.8 mmol), followed by degassing by filling with nitrogen. Add Pd(OAc) to it 2 (51.6 mg, 0.23 mmol). After filling with nitrogen, the reaction mixture was stirred at 130 °C for 18 hours. After the reaction was complete, the mixture was then washed with EA / H 2 O extraction. The organic layer was wash...
Embodiment 2
[0138] Example 2: 5-chloro-N2-(2-isopropoxy-5-methyl-4-(1,2,3,6-tetrahydropyridin-4-yl)phenyl)-N4-(2 Preparation of -(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine
[0139] Step 1: 4-(4-((5-Chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-5-isopropoxy-2- Preparation of tert-butyl methylphenyl)-5,6-dihydropyridine-1(2H)-carboxylate
[0140]
[0141] 2,5-dichloro-N-(2-(isopropylsulfonyl)phenyl)pyrimidin-4-amine (1 g, 2.88 mmol) was dissolved in 20 ml of THF, and 4-(4-amino - tert-butyl 5-isopropoxy-2-methylphenyl)-5,6-dihydropyridine-1(2H)-carboxylate (955 mg, 2.88 mmol). Add 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (167 mg, 0.288 mmol) and Cs in sequence 2 CO 3 (2.8 g, 8.64 mmol), followed by degassing by filling with nitrogen. Add Pd(OAc) to it 2 (32 mg, 0.144 mmol). After filling with nitrogen, the reaction mixture was stirred at 130 °C for 18 hours. After the reaction was complete, the mixture was then washed with EA / H 2 O extraction. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com