Bubble-type microneedle and preparation method therefor
A technology of microneedles and air bubbles, applied in the direction of microneedles, needles, and other medical devices, to achieve the effect of improving drug loading efficiency, simple method, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
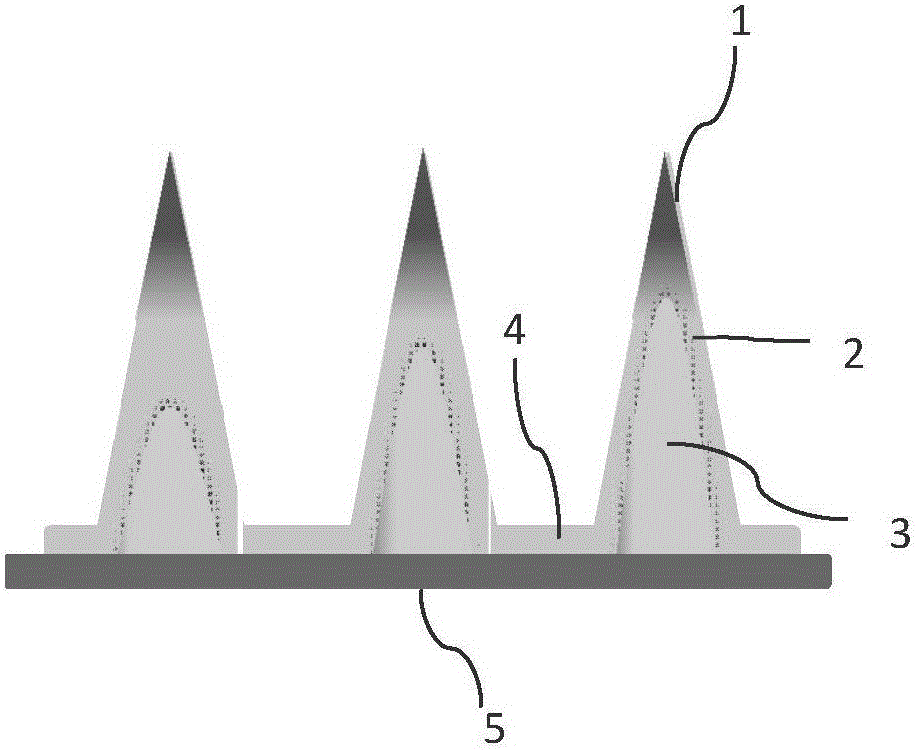
[0018] Prepare PVA air bubble microneedles and common PVA microneedles.
[0019] The material for preparing microneedles is polyvinyl alcohol (PVA), and the model drug used is sulforhodamine B. The specific steps are as follows: first, prepare a PVA aqueous solution with a mass percentage of 20 wt% as a microneedle preparation solution; prepare sulforhodamine B with a mass concentration of 1 mg / mL as a model drug solution;
[0020] The steps of the drug loading process are: under vacuum conditions, take the model drug solution and apply it on the microneedle template, after 5 minutes, recover the excess drug solution, and continue to dry in a vacuum environment for 10 minutes;
[0021] The steps for preparing ordinary PVA microneedles are as follows: after the drug loading process is completed, a layer of microneedle preparation solution with a thickness of 2000-3000 μm is coated on the surface of the microneedle template, and vacuum filling takes 45 minutes; the microneedle b...
Embodiment 2
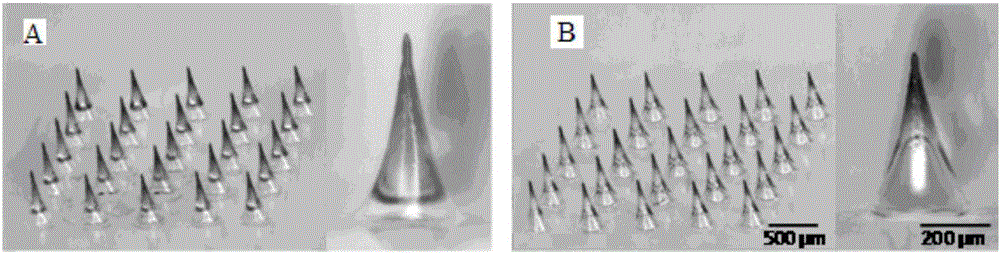
[0026] Bubble-type microneedles with different volume bubble structures were prepared.
[0027] The preparation liquid used for preparing the microneedles is a PVA aqueous solution with a mass percentage of 10-30 wt%, and the viscosity of each group of PVA solutions is measured, and the model drug used is sulforhodamine B. Specific steps are as follows:
[0028]Preparation concentration is 10wt%, 15wt%, 20wt%, 25wt%, the PVA aqueous solution of 30wt% is used as microneedle preparation liquid; Preparation mass concentration is sulfonyl rhodamine B of 1mg / mL as model drug solution; Microneedle preparation basic procedure and Similar to Example 1, different concentrations of PVA solutions were selected when coating the thin layer of microneedle preparation solution, and other conditions were the same. Finally, the microneedles were released from the mold to obtain bubble-type microneedles with different volume bubble structures. Similarly, corresponding ordinary microneedles we...
Embodiment 3
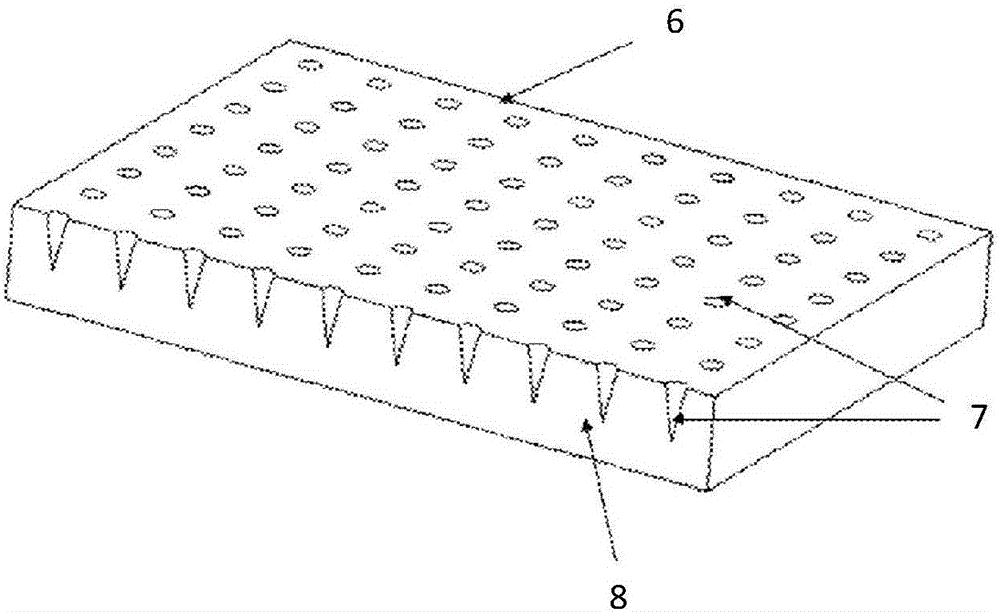
[0033] Preparation of sodium hyaluronate (HA) air bubble microneedles loaded with hepatitis B vaccine:
[0034] The material for preparing the microneedle is sodium hyaluronate, the drug used is hepatitis B vaccine, and the stabilizer used is sucrose. Concrete preparation steps are as follows:
[0035] First, prepare a microneedle preparation solution containing 15wt% HA and 10wt% sucrose; prepare a hepatitis B vaccine solution with a mass concentration of 2 mg / mL;
[0036] The steps of preparing the microneedle with HA bubble structure loaded with hepatitis B vaccine are as follows: under vacuum condition, take the vaccine solution and apply it on the template containing 10×10 microneedle array, after 5 minutes, recover the excess vaccine solution, continue to vacuum Dry for 10 minutes; apply a layer of microneedle preparation solution with a thickness of 100 μm on the surface of the microneedle template, and vacuum fill it for 30 minutes; after the microneedle body and its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com